Phosphorylation of myosin II regulatory light chain by ZIP kinase is responsible for cleavage furrow ingression during cell division in mammalian cultured cells.
Kosuke Hosoba, Satoshi Komatsu, Mitsuo Ikebe, Manato Kotani, Xiao Wenqin, Taro Tachibana, Hiroshi Hosoya, Kozue Hamao
文献索引:Biochem. Biophys. Res. Commun. 459(4) , 686-91, (2015)
全文:HTML全文
摘要
Zipper-interacting protein kinase (ZIPK) is known to regulate several functions such as apoptosis, smooth muscle contraction, and cell migration. While exogenously expressed GFP-ZIPK localizes to the cleavage furrow, role of ZIPK in cytokinesis is obscure. Here, we show that ZIPK is a major MRLC kinase during mitosis. Moreover, ZIPK siRNA-mediated knockdown causes delay of cytokinesis. The delay in cytokinesis of ZIPK-knockdown cells was rescued by the exogenous diphosphorylation-mimicking MRLC mutant. Taken together, these findings suggest that ZIPK plays a role in the progression and completion of cytokinesis through MRLC phosphorylation. Copyright © 2015 Elsevier Inc. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
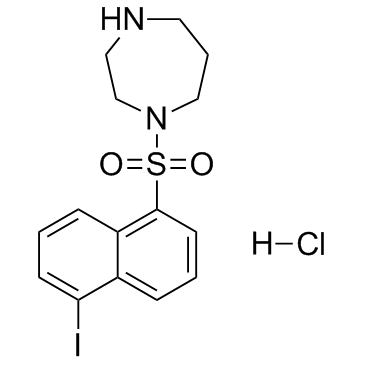 |
ML-7盐酸盐
CAS:110448-33-4 |
C15H18ClIN2O2S |
|
Myosin II controls cellular branching morphogenesis and migr...
2015-02-01 [Nat. Cell Biol. 17(2) , 137-47, (2015)] |
|
Directional bleb formation in spherical cells under temperat...
2015-07-21 [Biophys. J. 109 , 355-64, (2015)] |
|
MAPK3/1 (ERK1/2) and Myosin Light Chain Kinase in Mammalian ...
2015-06-01 [Biol. Reprod. 92 , 146, (2015)] |
|
Pseudomonas fluorescens alters the intestinal barrier functi...
2015-03-01 [Inflamm. Bowel Dis. 21(3) , 543-55, (2015)] |
|
ML-7 amplifies the quinocetone-induced cell death through ak...
2016-01-01 [Toxicol. Mech. Methods 26 , Nov-21, (2016)] |