N6-异戊烯基腺嘌呤
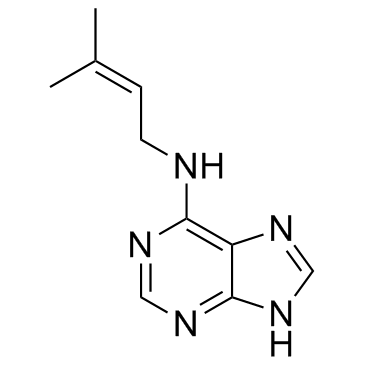
N6-异戊烯基腺嘌呤结构式

|
常用名 | N6-异戊烯基腺嘌呤 | 英文名 | 6-(γ,γ-Dimethylallylamino)purine |
|---|---|---|---|---|
| CAS号 | 2365-40-4 | 分子量 | 203.244 | |
| 密度 | 1.3±0.1 g/cm3 | 沸点 | 477.1±30.0 °C at 760 mmHg | |
| 分子式 | C10H13N5 | 熔点 | N/A | |
| MSDS | 中文版 美版 | 闪点 | 242.4±24.6 °C |
N6-异戊烯基腺嘌呤用途用作植物生长调节剂 |
| 中文名 | 烯腺嘌呤 |
|---|---|
| 英文名 | N6-dimethylallyladenine |
| 中文别名 | 6-(γ,γ-二甲基烯丙基氨基)嘌呤 | N6-异戊烯基腺嘌呤 | 6-(y,y-二甲基烯丙基氨基)嘌呤 | N6-(2-异戊烯基)腺嘌呤 |
| 英文别名 | 更多 |
| 描述 | 6-(γ,γ-Dimethylallylamino)purine 是一种植物生长调节剂。 |
|---|---|
| 相关类别 | |
| 体外研究 | 衍生紫外光谱数据显示,植物生长物质6-(γ,γ-二甲基烯丙基氨基)嘌呤(N6-(δ-异戊烯基)腺嘌呤,i6Ade)和吲哚乙酸(IAA)可与酵母醇脱氢酶(ADH)结合,影响辅酶-酶结合。在固定的乙醇浓度(27.8和111.1 mM)和不同的NAD +浓度(0.033-2 mM),以及固定水平的辅酶(0.67和2 mM)和不同浓度的乙醇(1.4-111.1 mM)时, i6Ade和IAA显着抑制乙醇氧化速率。 ADH反应的动力学受两个抑制常数(Ki和Ki')的影响,这两个抑制常数分别对应于复合物EI和ESI的解离常数。对于i6Ade,Ki = 0.52±0.06mM,Ki'= 0.74±0.07mM,对于IAA,Ki = 0.88±0.03mM,Ki'= 0.99±0.02mM [1]。 |
| 参考文献 |
| 密度 | 1.3±0.1 g/cm3 |
|---|---|
| 沸点 | 477.1±30.0 °C at 760 mmHg |
| 分子式 | C10H13N5 |
| 分子量 | 203.244 |
| 闪点 | 242.4±24.6 °C |
| 精确质量 | 203.117096 |
| PSA | 66.49000 |
| LogP | 2.31 |
| InChIKey | HYVABZIGRDEKCD-UHFFFAOYSA-N |
| SMILES | CC(C)=CCNc1ncnc2nc[nH]c12 |
| 外观性状 | 白色至灰白色粉末 |
| 蒸汽压 | 0.0±1.2 mmHg at 25°C |
| 折射率 | 1.683 |
| 储存条件 | -20°储存。 |
| 分子结构 | 1、 摩尔折射率:60.84 2、 摩尔体积(m3/mol):160.4 3、 等张比容(90.2K):454.5 4、 表面张力(dyne/cm):64.4 5、 极化率(10 -24cm 3):24.12 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:2 3.氢键受体数量:4 4.可旋转化学键数量:3 5.互变异构体数量:8 6.拓扑分子极性表面积66.5 7.重原子数量:15 8.表面电荷:0 9.复杂度:236 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1. 性状:未确定 2. 密度(g/L,25ºC):未确定 3. 相对蒸汽密度(g/mL,空气=1):未确定 4. 熔点(ºC):未确定 5. 沸点(ºC,常压):未确定 6. 沸点(ºC 25mmHg):未确定 7. 折射率(nD20):未确定 8. 闪点(ºC):未确定 9. 比旋光度():未确定 10. 自燃点或引燃温度(ºC):未确定 11. 蒸气压(Pa,20ºC):未确定 12. 饱和蒸气压(kPa,20ºC):未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC):未确定 15. 临界压力(KPa):未确定 16. 油水(辛醇/水)分配系数的对数值:未确定 17. 爆炸上限(%,V/V):未确定 18. 爆炸下限(%,V/V):未确定 19. 溶解性:未确定 |
| 个人防护装备 | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| 危害码 (欧洲) | Xi |
| 危险品运输编码 | NONH for all modes of transport |
| 海关编码 | 2934993000 |
| N6-异戊烯基腺嘌呤上游产品 8 | |
|---|---|
| N6-异戊烯基腺嘌呤下游产品 5 | |
| 海关编码 | 2933990090 |
|---|---|
| 中文概述 | 2933990090. 其他仅含氮杂原子的杂环化合物. 增值税率:17.0%. 退税率:13.0%. 监管条件:无. 最惠国关税:6.5%. 普通关税:20.0% |
| 申报要素 | 品名, 成分含量, 用途, 乌洛托品请注明外观, 6-己内酰胺请注明外观, 签约日期 |
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Synthesis, characterization and biological activity of ring-substituted 6-benzylamino-9-tetrahydropyran-2-yl and 9-tetrahydrofuran-2-ylpurine derivatives
Bioorg. Med. Chem. 17 , 1938-47, (2009) Synthesis of 9-tetrahydropyran-2-yl and 9-tetrahydrofuran-2-yl benzylaminopurines, their stability, cytokinin activity, perception by cytokinin receptors, degradation by cytokinin oxidase/dehydrogenas... |
|
|
Thidiazuron influences the endogenous levels of cytokinins and IAA during the flowering of isolated shoots of Dendrobium.
J. Plant Physiol. 163(11) , 1126-34, (2006) This study reports the effects of thidiazuron (TDZ) on the endogenous levels of indoleacetic acid (IAA), zeatin, zeatin riboside ([9R]Z), isopentenyladenine and isopentenyladenosine ([9R]iP) as well a... |
|
|
Functional identification of OsHk6 as a homotypic cytokinin receptor in rice with preferential affinity for iP.
Plant Cell Physiol. 53(7) , 1334-43, (2012) Cytokinins are involved in key developmental processes in rice (Oryza sativa), including the regulation of cell proliferation and grain yield. However, the in vivo action of histidine kinases (OsHks),... |
|
实验名称:Binding affinity to N-terminal His-tagged human CDK2 expressed in Escherichia coli BL...
来源:ChEMBL
靶标:Cyclin-dependent kinase 2
External Id:CHEMBL3757901
|
|
实验名称:Assays to identify small molecules inhibitory for eIF4E expression
来源:13133
靶标:N/A
External Id:20160513eIF4E
|
|
实验名称:Cell survival assay for modulators of telomere damage signalling
来源:15378
靶标:N/A
External Id:TELO_02
|
|
实验名称:Discovery of Small Molecules to Inhibit Human Cytomegalovirus Nuclear Egress
来源:ICCB-Longwood/NSRB Screening Facility, Harvard Medical School
靶标:HCMV UL50
External Id:HMS1262
|
|
实验名称:Experimentally measured binding affinity data (Ki) for protein-ligand complexes deriv...
来源:Shanghai Institute of Organic Chemistry
靶标:N/A
External Id:PDBbind-Ki for protein-ligand complexes
|
|
实验名称:A screen for compounds that inhibit the activity of LtaS in Staphylococcus aureus
来源:ICCB-Longwood/NSRB Screening Facility, Harvard Medical School
External Id:HMS979
|
|
实验名称:A screen for compounds that inhibit viral RNA polymerase binding and polymerization a...
来源:ICCB-Longwood/NSRB Screening Facility, Harvard Medical School
靶标:Chain A, Poliovirus Polymerase With Gtp
External Id:HMS750
|
|
实验名称:Ratio Kcat to Km for Zea mays CKX1 receptor
来源:ChEMBL
靶标:Cytokinin dehydrogenase 1
External Id:CHEMBL956875
|
|
实验名称:NCATS Parallel Artificial Membrane Permeability Assay (PAMPA) Profiling
来源:NCGC
靶标:N/A
External Id:ADME-PAMPA1
|
| 6-(y,y-Dimethylallylamino)purine |
| 6-isopentenyladenine |
| MFCD00132998 |
| N-(3-Methyl-2-buten-1-yl)-7H-purin-6-amine |
| N-(3-Methylbut-2-en-1-yl)-1H-purin-6-amin |
| iPeAde |
| 6-[(3-methylbut-2-en-1-yl)amino]-9H-purine |
| N-(3-methylbut-2-enyl)-7H-purin-6-amine |
| 6-[(3-Methyl-2-butenyl)amino]purine |
| N(6)-dimethylallyladenine |
| N6-[(3-methylbut-2-en-1-yl)amino]purine |
| N6-ISOPENTENYLADENINE (iP) |
| N6-(2-Isopentenyl)-adenosin |
| N-(3-methylbut-2-en-1-yl)-9H-purin-6-amine |
| N6-Isopentenyladenine |
| N6-(2-Isopentenyl)adenine |
| N6-(2-Isopentenyl)-adenine |
| N6-(δ 2-Isopentenyl)-adenine |
| Isopentenyl adenine |
| N6-iPeAde |
| N-(3-methylbut-2-en-1-yl)-1H-purin-6-amine |
| N6-(3-methylbut-2-enyl)adenine |
| isopentenyladenine |
| enadenine |
| TRIACANTHINE |
| N-Isopentenyladenine |
| N6-2-isopentenyladenine |
| N-(3-methylbut-2-en-1-yl)-7H-purin-6-amine |
| N-(3-methylbut-2-enyl)adenine |
| Isopentenyl-Adenine |
| 2-iP N6-(2-Isopentenyl)adenine |
| 2iP |
| N6-(delta 2-Isopentenyl)-adenine |
| N-(3-Methyl-2-buten-1-yl)-3H-purin-6-amine |
| N-(3-methyl-2-buten-1-yl)-1H-purin-6-amine |
| 6-(γ,γ-Dimethylallylamino)purine |

 CAS号29911-54-4
CAS号29911-54-4 CAS号26728-58-5
CAS号26728-58-5 CAS号87-42-3
CAS号87-42-3 CAS号56329-06-7
CAS号56329-06-7 CAS号556-82-1
CAS号556-82-1 CAS号5451-40-1
CAS号5451-40-1![[5-(6-acetamidopurin-9-yl)-3,4-diacetyloxy-oxolan-2-yl]methyl acetate结构式](https://image.chemsrc.com/caspic/032/7387-58-8.png) CAS号7387-58-8
CAS号7387-58-8 CAS号7724-76-7
CAS号7724-76-7 CAS号6025-53-2
CAS号6025-53-2 CAS号13822-06-5
CAS号13822-06-5 CAS号68-94-0
CAS号68-94-0![3H-Purine-3-butanoicacid, a-amino-6-[(3-methyl-2-buten-1-yl)amino]-,(aS)结构式](https://image.chemsrc.com/caspic/066/62061-49-8.png) CAS号62061-49-8
CAS号62061-49-8
