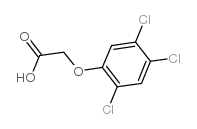
生态学数据:
1、 摩尔折射率:53.81
2、 摩尔体积(m3/mol):160.4
3、 等张比容(90.2K):433.1
4、 表面张力(dyne/cm):53.1
5、 极化率(10-24cm3):21.33
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
AJ8400000
-
CHEMICAL NAME :
-
Acetic acid, (2,4,5-trichlorophenoxy)-
-
CAS REGISTRY NUMBER :
-
93-76-5
-
BEILSTEIN REFERENCE NO. :
-
2055620
-
LAST UPDATED :
-
199712
-
DATA ITEMS CITED :
-
91
-
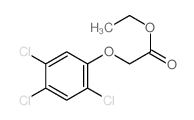
MOLECULAR FORMULA :
-
C8-H5-Cl3-O3
-
MOLECULAR WEIGHT :
-
255.48
-
WISWESSER LINE NOTATION :
-
QV1OR BG DG EG
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
300 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1535 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
500 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
242 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
100 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
381 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - hamster
-
DOSE/DURATION :
-
425 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Bird - chicken
-
DOSE/DURATION :
-
310 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - gastritis Behavioral - somnolence (general depressed activity) Liver - fatty liver degeneration
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - species unspecified
-
DOSE/DURATION :
-
500 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
196 mg/kg/28W-I
-
TOXIC EFFECTS :
-
Liver - fatty liver degeneration
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
700 mg/kg/90D-I
-
TOXIC EFFECTS :
-
Blood - changes in other cell count (unspecified) Nutritional and Gross Metabolic - weight loss or decreased weight gain Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
3379 mg/kg/33W-C
-
TOXIC EFFECTS :
-
Tumorigenic - neoplastic by RTECS criteria Kidney, Ureter, Bladder - tumors Skin and Appendages - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
215 mg/kg
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Lungs, Thorax, or Respiration - tumors Blood - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
100 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
6 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - behavioral
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
714 mg/kg
-
SEX/DURATION :
-
multigenerations
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - live birth index (measured after birth) Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
27600 ug/kg
-
SEX/DURATION :
-
female 10-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
100 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
500 mg/kg
-
SEX/DURATION :
-
female 6-10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
300 mg/kg
-
SEX/DURATION :
-
female 13-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
27600 ug/kg
-
SEX/DURATION :
-
female 10-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - gastrointestinal system Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
278 mg/kg
-
SEX/DURATION :
-
female 10-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
60 mg/kg
-
SEX/DURATION :
-
female 10-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
200 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - stillbirth
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
6250 ug/kg
-
SEX/DURATION :
-
male 10 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - prostate, seminal vesicle, Cowper's gland, accessory glands Reproductive - Paternal Effects - other effects on male
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
300 mg/kg
-
SEX/DURATION :
-
female 12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
900 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
150 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1 gm/kg
-
SEX/DURATION :
-
female 8-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - live birth index (measured after birth) Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
1 gm/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Maternal Effects - other effects Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
1 gm/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
1 gm/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
450 mg/kg
-
SEX/DURATION :
-
female 6-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
200 mg/kg
-
SEX/DURATION :
-
female 7-11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
500 mg/kg
-
SEX/DURATION :
-
female 7-11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
Specific locus test
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TYPE OF TEST :
-
Sex chromosome loss and nondisjunction
-
TYPE OF TEST :
-
Sex chromosome loss and nondisjunction
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TYPE OF TEST :
-
Cytogenetic analysis
MUTATION DATA
-
TEST SYSTEM :
-
Rodent - gerbil
-
REFERENCE :
-
ECBUDQ Ecological Bulletins. (Editorial Service of FRN, Box 6710, S-11385, Stockholm, Sweden) No.19- 1975- Volume(issue)/page/year: 27,182,1978 *** REVIEWS *** ACGIH TLV-Not classifiable as a human carcinogen DTLVS* The Threshold Limit Values (TLVs) and Biological Exposure Indices (BEIs) booklet issues by American Conference of Governmental Industrial Hygienists (ACGIH), Cincinnati, OH, 1996 Volume(issue)/page/year: TLV/BEI,1997 ACGIH TLV-TWA 10 mg/m3 DTLVS* The Threshold Limit Values (TLVs) and Biological Exposure Indices (BEIs) booklet issues by American Conference of Governmental Industrial Hygienists (ACGIH), Cincinnati, OH, 1996 Volume(issue)/page/year: TLV/BEI,1997 IARC Cancer Review:Human Limited Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 41,357,1986 IARC Cancer Review:Animal Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 15,273,1977 TOXICOLOGY REVIEW RREVAH Residue Reviews. (Springer-Verlag New York, Inc., Service Center, 44 Hartz Way, Secaucus, NJ 07094) V.1- 1962- Volume(issue)/page/year: 59,1,1975 TOXICOLOGY REVIEW ARPAAQ Archives of Pathology. (Chicago, IL) V.5(3)-50(3), 1928-50; V.70-99, 1960-75. For publisher information, see APLMAS. Volume(issue)/page/year: 94,125,1972 TOXICOLOGY REVIEW DTTIAF Deutsche Tieraerztliche Wochenschrift. (Hanover, Fed. Rep. Ger.) V.1-77, 1893-1970. Volume(issue)/page/year: 80,485,1973 TOXICOLOGY REVIEW RREVAH Residue Reviews. (Springer-Verlag New York, Inc., Service Center, 44 Hartz Way, Secaucus, NJ 07094) V.1- 1962- Volume(issue)/page/year: 56,107,1975 TOXICOLOGY REVIEW ECMAAI Economie et Medecine Animales. (Paris, France) V.1-17, 1960-76. Discontinued. Volume(issue)/page/year: 14,141,1973 TOXICOLOGY REVIEW BIOGAL Biologico. (Instituto Biologica, Av. Cons. Rodriques Alves, 1252, CEP 04014, Sao Paulo, Brazil) V.1- 1935- Volume(issue)/page/year: 40(2),44,1974 TOXICOLOGY REVIEW STEVA8 Science of the Total Environment. (Elsevier Science Pub. B.V., POB 211, 1000 AE Amsterdam, Netherlands) V.1- 1972- Volume(issue)/page/year: 2,305,1974 TOXICOLOGY REVIEW EVSRBT Environmental Science Research. (Plenum Pub. Corp., 233 Spring St., New York, NY 10013) V.1- 1972- Volume(issue)/page/year: 2,708,1973 TOXICOLOGY REVIEW VHTODE Veterinary and Human Toxicology. (American College of Veterinary and Comparative Toxicology, Publication Office, Comparative Toxicology, Manhattan, KS 66506) V.19- 1977- Volume(issue)/page/year: 22,31,1980 *** U.S. STANDARDS AND REGULATIONS *** MSHA STANDARD-air:TWA 10 mg/m3 DTLVS* The Threshold Limit Values (TLVs) and Biological Exposure Indices (BEIs) booklet issues by American Conference of Governmental Industrial Hygienists (ACGIH), Cincinnati, OH, 1996 Volume(issue)/page/year: 3,242,1971 OSHA PEL (Gen Indu):8H TWA 10 mg/m3 CFRGBR Code of Federal Regulations. (U.S. Government Printing Office, Supt. of Documents, Washington, DC 20402) Volume(issue)/page/year: 29,1910.1000,1994 OSHA PEL (Construc):8H TWA 10 mg/m3 CFRGBR Code of Federal Regulations. (U.S. Government Printing Office, Supt. of Documents, Washington, DC 20402) Volume(issue)/page/year: 29,1926.55,1994 OSHA PEL (Shipyard):8H TWA 10 mg/m3 CFRGBR Code of Federal Regulations. (U.S. Government Printing Office, Supt. of Documents, Washington, DC 20402) Volume(issue)/page/year: 29,1915.1000,1993 OSHA PEL (Fed Cont):8H TWA 10 mg/m3 CFRGBR Code of Federal Regulations. (U.S. Government Printing Office, Supt. of Documents, Washington, DC 20402) Volume(issue)/page/year: 41,50-204.50,1994 *** OCCUPATIONAL EXPOSURE LIMITS *** OEL-AUSTRALIA:TWA 10 mg/m3 JAN 1993 OEL-BELGIUM:TWA 10 mg/m3 JAN 1993 OEL-DENMARK:TWA 5 mg/m3;Skin JAN 1993 OEL-FRANCE:TWA 10 mg/m3 JAN 1993 OEL-GERMANY:TWA 10 mg/m3;Skin JAN 1993 OEL-HUNGARY:TWA 1 mg/m3;STEL 2 mg/m3;Skin JAN 1993 OEL-THE NETHERLANDS:TWA 10 mg/m3 JAN 1993 OEL-THE PHILIPPINES:TWA 10 mg/m3 JAN 1993 OEL-SWITZERLAND:TWA 10 mg/m3;STEL 50 mg/m3;Skin JAN 1993 OEL-THAILAND:TWA 10 mg/m3 JAN 1993 OEL-UNITED KINGDOM:TWA 10 mg/m3;STEL 20 mg/m3 JAN 1993 OEL IN BULGARIA, COLOMBIA, JORDAN, KOREA check ACGIH TLV OEL IN NEW ZEALAND, SINGAPORE, VIETNAM check ACGIH TLV *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH RECOMMENDED EXPOSURE LEVEL (REL) : NIOSH REL TO 2,4,5-T-air:10H TWA 10 mg/m3 REFERENCE : NIOSH* National Institute for Occupational Safety and Health, U.S. Dept. of Health, Education, and Welfare, Reports and Memoranda. Volume(issue)/page/year: DHHS #92-100,1992 NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOHS - National Occupational Hazard Survey (1974) NOHS Hazard Code - 73900 No. of Facilities: 177 (estimated) No. of Industries: 2 No. of Occupations: 3 No. of Employees: 1560 (estimated)
|