13478-18-7
| 中文名 | 三氯化钼 |
|---|---|
| 英文名 | molybdenum trichloride |
| 中文别名 | 氯化钼(III) |
| 英文别名 |
trichloromolybdenum
Molybdenum(III) chloride MoCl3 MFCD00014214 molybdenum trichloride EINECS 236-775-9 Molybdenum(3+) trichloride trichloridomolybdenum |
| 密度 | 3.58 g/mL at 25 °C(lit.) |
|---|---|
| 熔点 | 1027°C |
| 分子式 | Cl3Mo |
| 分子量 | 202.299 |
| 精确质量 | 202.811966 |
| LogP | 2.06850 |
| 外观性状 | Powder | Dark red |
| 蒸汽压 | 33900mmHg at 25°C |
| 储存条件 | 常温密闭,阴凉通风干燥惰性气体下 |
| 稳定性 | 常温常压下稳定 避免的物料 水分/潮湿 空气 氧化物。MoCl3有两种晶形,即Cl原子处于六方密堆积和立方密堆积的排列中,其中成对的钼原子穿过一共用边,处于相邻八面体空穴中,其间距为0.276nm。这说明了MoCl3中有MoMo键的存在,同时磁性的测定结果也与此相符。二聚离子[Mo2Cl9]3-呈抗磁性,并可能具有像[W2Cl9]3-那样的结构,即由两个共享一个面的畸变的MoCl6八面体组成,其中金属金属键矩较短,也说明了存在金属金属键。 当隔绝空气时这种暗红色的化合物是稳定的。它能被潮湿空气逐渐地水解和氧化。它不溶于水、稀盐酸、丙酮、四氯化碳和苯。它在乙醚、异丙醇和吡啶中有微小的溶解度,但是这个化合物能被稀硝酸完全溶解,或许是生成了某些更高度氧化的物种。差热分析研究表明氯化钼(Ⅲ)在温度高于410℃时歧化成MoCl2和MoCl4(冷却时生成MoCl3+MoCl5)[75],α-MoCl3和β-MoCl3的结构(两者都是单斜晶系的)都曾被确定过 |
| 水溶解性 | H2O: insoluble(lit.) | Soluble in nitric acid and sulfuric acid. Insoluble in water and dilute hydrochloric acid. Slightly soluble in ethanol and diethyl ether. |
| 分子结构 | 1、摩尔折射率:无可用的 2、摩尔体积(cm3/mol):无可用的 3、等张比容(90.2K):无可用的 4、表面张力(dyne/cm):无可用的 5、介电常数:无可用的 6、极化率(10-24cm3):无可用的 7、单一同位素质量:202.811965 Da 8、标称质量:203 Da 9、平均质量:202.299 Da |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:0 3.氢键受体数量:0 4.可旋转化学键数量:0 5.互变异构体数量:无 6.拓扑分子极性表面积0 7.重原子数量:4 8.表面电荷:0 9.复杂度:8 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1. 性状:紫铜色的单斜晶系结晶。 2. 密度(g/mL,25/4℃):3.74 3. 相对蒸汽密度(g/mL,空气=1):未确定 4. 熔点(ºC):未确定 5. 沸点(ºC,常压):未确定 6. 沸点(ºC,5.2kPa):未确定 7. 折射率:未确定 8. 闪点(ºC):未确定 9. 比旋光度(º):未确定 10. 自燃点或引燃温度(ºC):未确定 11. 蒸气压(kPa,25ºC):未确定 12. 饱和蒸气压(kPa,60ºC):未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC):未确定 15. 临界压力(KPa):未确定 16. 油水(辛醇/水)分配系数的对数值:未确定 17. 爆炸上限(%,V/V):未确定 18. 爆炸下限(%,V/V):未确定 19. 溶解性:分解 |
|
Section 1: Product Identification Chemical Name:Molybdenum(III) chloride (99.5%-Mo) CAS Registry Number:13478-18-7 Formula:MoCl3 EINECS Number:236-775-9 Chemical Family:metal halide Synonym:Molybdenum trichloride
Section 2: Composition and Information on Ingredients IngredientCAS NumberPercentACGIH (TWA)OSHA (PEL) Title Compound13478-18-7100%0.5mg/m35mg/m3 Section 3: Hazards Identification Emergency Overview:Corrosive to skin, eyes and respiratory tract. Inhalation of dust leads to burning of the respiratory tract. Primary Routes of Exposure:Contact with skin and eyes. Inhalation of dust. Eye Contact:Corrosive to the eyes. Skin Contact:Causes burns to the skin. Inhalation:Inhalation of dust leads to chemical burns of the respiratory tract. Ingestion:No information is available on the physiological effects of ingestion. Acute Health Affects:Corrosive to skin, eyes and respiratory tract. Chronic Health Affects:No information available on long-term chronic effects. NTP:No IARC:No OSHA:No SECTION 4: First Aid Measures Immediately flush the eyes with copious amounts of water for at least 10-15 minutes. A victim may need Eye Exposure: assistance in keeping their eye lids open. Get immediate medical attention. Wash the affected area with water. Remove contaminated clothes if necessary. Seek medical assistance if Skin Exposure: irritation persists. Remove the victim to fresh air. Closely monitor the victim for signs of respiratory problems, such as difficulty Inhalation: in breathing, coughing, wheezing, or pain. In such cases seek immediate medical assistance. Seek medical attention immediately. Keep the victim calm. Give the victim water (only if conscious). Induce Ingestion: vomiting only if directed by medical personnel. SECTION 5: Fire Fighting Measures Flash Point:not applicable Autoignition Temperature:none Explosion Limits:none Extinguishing Medium:none required If involved in a fire, fire fighters should be equipped with a NIOSH approved positive pressure self-contained Special Fire Fighting Procedures: breathing apparatus and full protective clothing. Hazardous Combustion andnone Decomposion Products: Unusual Fire or Explosion Hazards: No unusual fire or explosion hazards. SECTION 6: Accidental Release Measures Small spills can be neutralized with powdered sodium bicarbonate, lime, or calcium carbonate and swept up. Spill and Leak Procedures:Large spills in areas not adequately ventilated will require an evacuation of site. Emergency response teams will require self-contained breathing apparatus. SECTION 7: Handling and Storage Handling and Storage:Store material in a tightly sealed container under an inert atmosphere of nitrogen or argon. SECTION 8: Exposure Controls and Personal Protection Eye Protection:Always wear approved safety glasses when handling a chemical substance in the laboratory. Skin Protection:Wear protective clothing gloves. Consult glove manufacturer to determine the proper type of glove. Ventilation:If possible, handle the material in an efficient fume hood. If in form of fine dust and ventilation is not available a respirator should be worn. The use of respirators Respirator: requires a Respirator Protection Program to be in compliance with 29 CFR 1910.134. Ventilation:If possible, handle the material in an efficient fume hood. Additional Protection:No additional protection required. SECTION 9: Physical and Chemical Properties Color and Form:-100 mesh purple xtl. Molecular Weight:202.32 Melting Point:dec. Boiling Point:no data Vapor Pressure:no data Specific Gravity:3.59 Odor:none Solubility in Water:reacts with water SECTION 10: Stability and Reactivity Stability:air sensitive, moisture sensitive Hazardous Polymerization:no hazardous polymerization Conditions to Avoid:contact with moisture Incompatibility:active metals Decomposition Products:none SECTION 11: Toxicological Information RTECS Data:Oral (insect-Drosophila melanogaster); Specific locus test: 10nmol/L. Carcinogenic Effects:no data Mutagenic Effects:Mutagen Tetratogenic Effects:no data SECTION 12: Ecological Information Ecological Information:No information available SECTION 13: Disposal Considerations Disposal:Dispose of according to local, state and federal regulations. SECTION 14: Transportation Shipping Name (CFR):Corrosive solids, N.O.S. Hazard Class (CFR):8 Additional Hazard Class (CFR):NA Packaging Group (CFR):II UN ID Number (CFR):UN# 1759 Shipping Name (IATA):Corrosive solid, N.O.S. Hazard Class (IATA):8 Additional Hazard Class (IATA):NA Packaging Group (IATA):II UN ID Number (IATA):UN# 1759 SECTION 15: Regulatory Information TSCA:Listed in the TSCA inventory. SARA (Title 313):Title compound not listed Second Ingredient:none SECTION 16 - ADDITIONAL INFORMATION N/A |
|
毒理学数据: 1、急性毒性:主要的刺激性影响: 在皮肤上面:在皮肤和粘膜上造成腐蚀性影响,刺激皮肤和粘膜; 在眼睛上面:强烈的腐蚀性影响,刺激的影响;没有已知的敏化影响。 生态学数据: 通常对水体是稍微有害的,不要将未稀释或大量产品接触地下水,水道或污水系统,未经政府许可勿将材料排入周围环境。 CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| 符号 |

GHS07 |
|---|---|
| 信号词 | Warning |
| 危害声明 | H315-H319-H335 |
| 警示性声明 | P261-P305 + P351 + P338 |
| 个人防护装备 | dust mask type N95 (US);Eyeshields;Gloves |
| 危害码 (欧洲) | Xi: Irritant; |
| 风险声明 (欧洲) | R36/37/38 |
| 安全声明 (欧洲) | S26;S37/S39 |
| 危险品运输编码 | UN3260 |
| WGK德国 | 3 |
| RTECS号 | QA4681400 |
| 包装等级 | III |
| 危险类别 | 8 |
|
~% 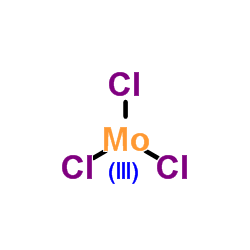
13478-18-7 |
| 文献:Ullmanns Encykl. Tech. Chem. 4th Ed., , vol. 17, p. 23 - 50 Mo: SVol.A3, 2.4.6.2, page 62 - 63 |
|
~% 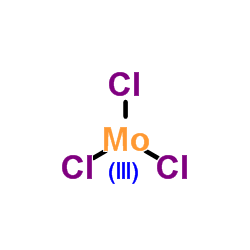
13478-18-7 |
| 文献:Journal fuer Praktische Chemie (Leipzig), , vol. 71, p. 453 - 453 |
|
~% 
13478-18-7 |
| 文献:Mo: SVol.A3, 2.7.27, page 182 - 183 |
|
~% 
13478-18-7 |
| 文献:Chemiker-Zeitung, , vol. 12, p. 5 - 5 Mo: MVol., 56, page 162 - 164 |
|
~% 
13478-18-7 |
| 文献:Journal of the Chemical Society, Dalton Transactions: Inorganic Chemistry (1972-1999), , p. 1197 - 1198 |
|
~% 
13478-18-7 |
| 文献:European Journal of Inorganic Chemistry, , # 10 p. 2699 - 2703 |
| 上游产品 6 | |
|---|---|
| 下游产品 1 | |