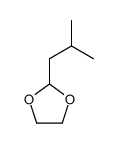
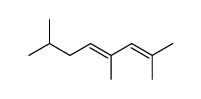
2256-01-1
| 中文名 | 2,4-二硝基苯腙异戊醛 |
|---|---|
| 英文名 | N-(3-methylbutylideneamino)-2,4-dinitroaniline |
| 中文别名 |
异戊醛-2,4-二硝基苯腙
异戊醛-DNPH 异戊醛2,4-二硝基苯腙 |
| 英文别名 |
(2,4-dinitrophenyl)hydrazone
Butanal,3-methyl-,2-(2,4-dinitrophenyl)hydrazone Isovaleraldehyde 2,4-Dinitrophenylhydrazone 3-methylbutanal (2,4-dinitrophenyl)hydrazone S0705 3-methyl-butanal I0477 isovaleraldehyde 2,4-dinitrophenylhydrazone Isovaleraldehyd-2,4-dinitrophenylhydrazon Isovaleraldehyde-DNPH |
| 密度 | 1.33g/cm3 |
|---|---|
| 沸点 | 406ºC at 760mmHg |
| 熔点 | 122-124ºC(lit.) |
| 分子式 | C11H14N4O4 |
| 分子量 | 266.25300 |
| 闪点 | 199.3ºC |
| 精确质量 | 266.10200 |
| PSA | 116.03000 |
| LogP | 4.06620 |
| 外观性状 | 固体;White to Yellow to Orange powder to crystal |
| 蒸汽压 | 8.44E-07mmHg at 25°C |
| 折射率 | 1.594 |
| 储存条件 | 0-6°C储存 |
| 分子结构 | 1、 摩尔折射率:67.74 2、 摩尔体积(m3/mol):199.6 3、 等张比容(90.2K):539.6 4、 表面张力(dyne/cm):53.3 5、 极化率(10 -24cm 3):26.85 |
| 计算化学 | 1、 疏水参数计算参考值(XlogP):2.1 2、 氢键供体数量:1 3、 氢键受体数量:6 4、 可旋转化学键数量:4 5、 互变异构体数量: 6、 拓扑分子极性表面积(TPSA):111 7、 重原子数量:19 8、 表面电荷:0 9、 复杂度:348 10、同位素原子数量:0 11、确定原子立构中心数量:0 12、不确定原子立构中心数量:0 13、确定化学键立构中心数量:1 14、不确定化学键立构中心数量:0 15、共价键单元数量:1 |
| 更多 | 1. 性状:未确定 2. 密度(g/L,25ºC):未确定 3. 相对蒸汽密度(g/mL,空气=1):未确定 4. 熔点(ºC):122-124 5. 沸点(ºC,常压):未确定 6. 沸点(ºC 0.1mmHg):未确定 7. 折射率(nD20):未确定 8. 闪点(ºF):未确定 9. 比旋光度():未确定 10. 自燃点或引燃温度(ºC):未确定 11. 蒸气压(Pa,25ºC):未确定 12. 饱和蒸气压(kPa,20ºC):未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC):未确定 15. 临界压力(KPa):未确定 16. 油水(辛醇/水)分配系数的对数值:未确定 17. 爆炸上限(%,V/V):未确定 18. 爆炸下限(%,V/V):未确定 19. 溶解性:未确定 |
|
Section I.Chemical Product and Company Identification Chemical NameIsovaleraldehyde 2,4-Dinitrophenylhydrazone (1mg/ml as Aldehyde in Ethyl Acetate) [for Offensive Odors Analysis] (1ml*5) Portland OR SynonymNot available. Chemical FormulaC11H14N4O4 CAS Number2256-01-1
141-78-6 (Ethyl Acetate) Section II.Composition and Information on Ingredients Toxicology Data Chemical NameCAS Number Percent (%)TLV/PEL Isovaleraldehyde 2,4-Dinitrophenylhydrazone0.11% Not available.(Ethyl Acetate) 2256-01-1 (1mg/ml as Aldehyde in Ethyl Acetate) [for Offensive Odors Analysis] (1ml*5) 141-78-6 (Ethyl 99.89% (EthylRat LD50 (oral) 5620 mg/kg Acetate)Mouse LD50 (oral) 4100 mg/kg Acetate) Rabbit LD50 (oral) 5500 mg/kg Rabbit LD50 (dermal) >20 mL/kg Rat LC50 (inhalation) 200 gm/m3 Mouse LC50 (inhalation) 45 gm/m3 Section III. Hazards Identification Acute Health EffectsIrritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening, or, occasionally, blistering. Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound. Chronic Health EffectsCARCINOGENIC EFFECTS : Not available. MUTAGENIC EFFECTS : Not available. TERATOGENIC EFFECTS : Not available. DEVELOPMENTAL TOXICITY: Not available. Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions. Section IV.First Aid Measures Eye ContactCheck for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical attention. Skin ContactIn case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing before reuse. Thoroughly clean shoes before reuse. Get medical attention. InhalationIf the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not improve. IngestionINDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat. Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic material was ingested; the absence of such signs, however, is not conclusive. Section V. Fire and Explosion Data 427°C (800.6°F) (Ethyl Acetate) Auto-Ignition FlammabilityFlammable. Flammable Limits Flash PointsLOWER: 2.2% UPPER: 11.5% (Ethyl -3°C (26.6°F). (Ethyl Acetate) Acetate) Combustion ProductsThese products are toxic carbon oxides (CO, CO 2), nitrogen oxides (NO, NO2). Fire Hazards Not available. Risks of explosion of the product in presence of mechanical impact: Not available. Explosion Hazards Risks of explosion of the product in presence of static discharge: Not available. Continued on Next Page Isovaleraldehyde 2,4-Dinitrophenylhydrazone (1mg/ml as Aldehyde in Ethyl Acetate) [for Offensive Odors Analysis] (1ml*5) Fire Fighting Media Flammable liquid. SMALL FIRE: Use DRY chemical powder. and Instructions LARGE FIRE: Use alcohol foam, water spray or fog. Consult with local fire authorities before attempting large scale fire-fighting operations. Section VI.Accidental Release Measures Spill CleanupFlammable material. Irritating material. Keep away from heat. Mechanical exhaust required. Stop leak if without risk. Absorb with DRY earth, sand or other Instructions non-combustible material. DO NOT touch spilled material. Prevent entry into sewers, basements or confined areas; dike if needed. Consult federal, state, and/or local authorities for assistance on disposal. Section VII. Handling and Storage Handling and StorageFLAMMABLE. IRRITANT. Keep away from heat. Mechanical exhaust required. Avoid excessive heat and light. Do not breathe gas/fumes/ vapor/spray. Information Section VIII. Exposure Controls/Personal Protection Engineering ControlsProvide exhaust ventilation or other engineering controls to keep the airborne concentrations of vapors below their respective threshold limit value. Ensure that eyewash station and safety shower is proximal to the work-station location. Splash goggles. Lab coat. Vapor respirator. Boots. Gloves. Suggested protective clothing might not be sufficient; Personal Protection consult a specialist BEFORE handling this product. Be sure to use a MSHA/NIOSH approved respirator or equivalent. Exposure LimitsNot available. Section IX. Physical and Chemical Properties Physical state @ 20°CLiquid.Solubility (Ethyl Acetate) One ml dissolves in 10ml water at 25°; 0.9 (water=1) (Ethyl Acetate)more soluble at lower and less soluble at Specific Gravity higher temps. Miscible with alcohol, acetone, chloroform, ether. Molecular WeightPartition Coefficient 266.25Not available. C4H8O2 =88.11 (Ethyl Acetate) Boiling Point 76.5 to 77.5°C (169.7 to 171.5°F) (EthylVapor Pressure73 mmHg (@ 20°C) (Ethyl Acetate) Acetate) Melting Point-84°C (-119.2°F) (Ethyl Acetate)Vapor Density3.04 (Air = 1) (Ethyl Acetate) 1.372 (Ethyl Acetate)VolatilityNot available. Refractive Index Critical TemperatureOdorCharacteristic fruity odor (Ethyl Acetate) Not available. ViscosityNot available.TasteNot available. Section X.Stability and Reactivity Data This material is stable if stored under proper conditions. (See Section VII for instructions) Stability Conditions of Instability Avoid excessive heat and light. Incompatibilities Reactive with strong oxidizing agents. Section XI. Toxicological Information RTECS NumberAH5425000 (Ethyl Acetate) Routes of ExposureEye Contact. Ingestion. Inhalation. (Ethyl Acetate) Toxicity Data Rat LD50 (oral) 5620 mg/kg Mouse LD50 (oral) 4100 mg/kg Rabbit LD50 (oral) 5500 mg/kg Rabbit LD50 (dermal) >20 mL/kg Rat LC50 (inhalation) 200 gm/m3 Mouse LC50 (inhalation) 45 gm/m3 Chronic Toxic EffectsCARCINOGENIC EFFECTS : Not available. MUTAGENIC EFFECTS : Not available. TERATOGENIC EFFECTS : Not available. DEVELOPMENTAL TOXICITY: Not available. Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions. Continued on Next Page Isovaleraldehyde 2,4-Dinitrophenylhydrazone (1mg/ml as Aldehyde in Ethyl Acetate) [for Offensive Odors Analysis] (1ml*5) Acute Toxic EffectsIrritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening, or, occasionally, blistering. Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound. Section XII.Ecological Information EcotoxicityNot available. Environmental Fatesoluble at lowe Ethyl acetate's production and use as a pharmaceutic aid, in artificial fruit essences, and as a solvent may result in its release to the environment through various waste streams. If released to air, a vapor pressure of 93 mm Hg at 25 deg C indicates ethyl acetate will exist solely as a vapor in the ambient atmosphere. Vapor-phase ethyl acetate will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 9.4 days. If released to soil, ethyl acetate is expected to have high mobility based upon an estimated Koc of 59. Volatilization from moist soil surfaces is expected to occur based upon a Henry's Law constant of 1.34X10-4 atm-cu m/mole. Ethyl acetate's vapor pressure indicates the potential for volatilization from dry soil surfaces exists. Biodegradation is expected to be an important process in both soil and water, based upon ethyl acetate's biodegradation in aqueous screening studies. 93% biodegradation was observed in a complete mix continuous-flow activated sludge system. 26.6 and 57.1% of ethyl acetate's theoretical BOD was reached in 5 days using the standard dilution method and seawater dilution method, respectively. If released into water, ethyl acetate is not expected to adsorb to suspended solids and sediment in water based on the estimated Koc. Volatilization from water surfaces is expected to be an important fate process based on ethyl acetate's Henry's Law constant. Estimated volatilization half-lives for a model river and model lake are 8.9 hours and 5.6 days, respectively. An estimated BCF of 3.2 suggests the potential for bioconcentration in aquatic organisms is low. Ethyl acetate's hydrolysis half-life at 25 deg C and pH 7 is 2.0 years. Occupational exposure to ethyl acetate may occur through inhalation and dermal contact with this compound at workplaces where ethyl acetate is produced or used or where adhesives, thinners, degreasers, paints, inks, and dome reagents are used. The general population may be exposed to ethyl acetate via inhalation of ambient air, ingestion of food and drinking water, or dermal contact with consumer products containing this compound. Section XIII. Disposal Considerations Waste DisposalRecycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when disposing of the substance. Section XIV. Transport Information DOT ClassificationDOT Class 9: Miscellaneous hazardous material PIN Number Proper Shipping Name Chemical Kits Packing Group (PG)III DOT Pictograms Section XV. Other Regulatory Information and Pictograms TSCA Chemical InventoryThis product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40 CFR 720.36 (C) for those products not on the inventory list: (EPA) (i) These products are supplied solely for use in research and development by or under the supervision of a technically qualified individual as defined in 40 CFR 720.0 et sec. (ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be supplied on an MSDS sheet. (Ethyl Acetate) This compound is ON the EPA Toxic Substances Control Act (TSCA) inventory list. WHMIS ClassificationCLASS B-2: Flammable liquid with a flash point lower than 37.8°C (100°F). (Canada)On DSL (Ethyl Acetate) EINECS Number (EEC) 205-500-4 (Ethyl Acetate) EEC Risk Statements R10- Flammable. R18- In use, may form flammable/explosive vapor-air mixture. R36/37/38- Irritating to eyes, respiratory system and skin. Japanese Regulatory Data ENCS No. 2-726 (Ethyl Acetate) Continued on Next Page Isovaleraldehyde 2,4-Dinitrophenylhydrazone (1mg/ml as Aldehyde in Ethyl Acetate) [for Offensive Odors Analysis] (1ml*5) SECTION 16 - ADDITIONAL INFORMATION N/A |
| 符号 |


GHS02, GHS07 |
|---|---|
| 信号词 | Danger |
| 危害声明 | H225-H302 + H332-H319 |
| 警示性声明 | P210-P305 + P351 + P338 |
| 危害码 (欧洲) | Xi,T,F |
| 风险声明 (欧洲) | 36/37/38-23/24/25-11-43 |
| 安全声明 (欧洲) | 26-36-45-27-16-36/37 |
| 危险品运输编码 | UN 1648 3/PG 2 |
| WGK德国 | 3 |
| 危险类别 | 1.0 |
| 海关编码 | 2928000090 |
|
~% 
2256-01-1 |
| 文献:Synthesis, , # 9 p. 692 - 693 |
|
~% 
2256-01-1 |
| 文献:Biochemical Journal, , vol. 31, p. 2022,2026 |
|
~% 
2256-01-1 |
| 文献:Sb. Statei Obshch. Khim., , p. 1418,1425 Chem.Abstr., , p. 4615 |
|
~% 
2256-01-1 |
| 文献:Sb. Statei Obshch. Khim., , p. 1418,1425 Chem.Abstr., , p. 4615 |
|
~% 
2256-01-1 |
| 文献:Sb. Statei Obshch. Khim., , p. 1418,1425 Chem.Abstr., , p. 4615 |
| 上游产品 4 | |
|---|---|
| 下游产品 0 | |
| 海关编码 | 2928000090 |
|---|---|
| 中文概述 | 2928000090 其他肼(联氨)及胲(羟胺)的有机衍生物。监管条件:无。增值税率:17.0%。退税率:9.0%。最惠国关税:6.5%。普通关税:20.0% |
| 申报要素 | 品名, 成分含量, 用途 |
| Summary | 2928000090 other organic derivatives of hydrazine or of hydroxylamine VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |