1317-60-8
| 中文名 | 赤铁矿 |
|---|---|
| 英文名 | Hematite |
| 英文别名 |
MFCD00011008
SIENNA miox[qr] HAEMATITE ironore EINECS 215-168-2 RAW UMBER ROUGE UMBER RAW MINETTE |
| 密度 | 5.24 |
|---|---|
| 熔点 | 1538 °C |
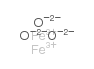
| 分子式 | Fe2O3 |
| 分子量 | 159.68800 |
| 闪点 | >230 °F |
| 精确质量 | 159.85500 |
| 外观性状 | 红色,无气味的粉末 |
| 储存条件 | 密封储存,储存于阴凉、干燥、通风良好的库房。远离不相容材料。 |
| 稳定性 | 常温常压稳定。避免与不相容材料,尘土接触。 与铝,二氧化碳,环氧乙烷,水合肼,次氯酸钙,五氟化溴,铯碳化物反应。 |
| 分子结构 | 1、摩尔折射率:无可用的 2、摩尔体积(cm3/mol):无可用的 3、等张比容(90.2K):无可用的 4、表面张力(dyne/cm):无可用的 5、介电常数:无可用的 6、极化率(10-24cm3):无可用的 7、单一同位素质量:159.854628 Da 8、标称质量:160 Da 9、平均质量:159.6882 Da |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:0 3.氢键受体数量:3 4.可旋转化学键数量:0 5.互变异构体数量:无 6.拓扑分子极性表面积3 7.重原子数量:5 8.表面电荷:0 9.复杂度:0 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:5 |
| 更多 | 1. 性状:外观为有金属光泽的紫褐色或紫灰色粉末 2. 密度(g/mL,25℃):5.24 3. 相对蒸汽密度(g/mL,空气=1):未确定 4. 熔点(ºC):1538 5. 沸点(ºC,常压):未确定 6. 沸点(ºC,3mmHg):未确定 7. 折射率(n20/D):未确定 8. 闪点(ºC):>110 9. 比旋光度(º):未确定 10. 自燃点或引燃温度(ºC):未确定 11. 蒸气压(mmHg,20ºC):1 12. 饱和蒸气压(kPa,25ºC):未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC):未确定 15. 临界压力(KPa):未确定 16. 油水(辛醇/水)分配系数的对数值:未确定 17. 爆炸上限(%,V/V):未确定 18. 爆炸下限(%,V/V):未确定 19. 溶解性:不溶于水 |
|
Section 1: Product Identification Chemical Name:Iron (III) oxide, red (Hematite) (99.8%-Fe) CAS Registry Number:1317-60-8 Formula:Fe2O3 EINECS Number:215-275-4 Chemical Family:metal oxide Synonym:Haematite, Iron ore. Red iron oxide. Ferric oxide.
Section 2: Composition and Information on Ingredients IngredientCAS NumberPercentACGIH (TWA)OSHA (PEL) Title Compound1317-60-8100%5mg/m310mg/m3 Section 3: Hazards Identification Emergency Overview:May be irritating to skin and eyes and respiratory tract. Primary Routes of Exposure:Ingestion, inhalation Eye Contact:May be a mild to severe irritant to the eyes. Skin Contact:May cause slight to mild irritation of the skin. Inhalation:Do not breath dust. Inhalation of powder may lead to irritation of the respiratory tract. Ingestion:No information available on the effects of ingestion. Acute Health Affects:May be irritating to skin, eyes, mucous membranes and respiratory tract. Do not breath dust. Chronic Health Affects:Prolonged inhalation of iron oxide dust can produce a benign pneumoconiosis known as siderosis. NTP:No IARC:No OSHA:No SECTION 4: First Aid Measures Immediately flush the eyes with copious amounts of water for at least 10-15 minutes. A victim may need Eye Exposure: assistance in keeping their eye lids open. Get immediate medical attention. Wash the affected area with water. Remove contaminated clothes if necessary. Seek medical assistance if Skin Exposure: irritation persists. Remove the victim to fresh air. Closely monitor the victim for signs of respiratory problems, such as difficulty Inhalation: in breathing, coughing, wheezing, or pain. In such cases seek immediate medical assistance. Seek medical attention immediately. Keep the victim calm. Give the victim water (only if conscious). Induce Ingestion: vomiting only if directed by medical personnel. SECTION 5: Fire Fighting Measures Flash Point:not applicable Autoignition Temperature:none Explosion Limits:none Extinguishing Medium:material is non-flammable Special Fire Fighting Procedures:No special fire-fighting procedures. The material is non-flammable. Hazardous Combustion andno hazardous decomposition products. Decomposion Products: Unusual Fire or Explosion Hazards: No unusual fire or explosion hazards. SECTION 6: Accidental Release Measures Spill and Leak Procedures:Small spills can be mixed with vermiculite or sodium carbonate and swept up. SECTION 7: Handling and Storage Handling and Storage:No special storage conditions. SECTION 8: Exposure Controls and Personal Protection Eye Protection:Always wear approved safety glasses when handling a chemical substance in the laboratory. Skin Protection:Wear protective clothing and gloves. Ventilation:Material may form a fine dust. If possible, handle the material in an efficient fume hood. If in the form of a fine dust and ventilation is not available, a respirator should be worn. The use of respirators Respirator: requires a Respiratory Protection Program to be in compliance with 29 CFR 1910.134. Ventilation:Material may form a fine dust. If possible, handle the material in an efficient fume hood. Additional Protection:No additional protection required. SECTION 9: Physical and Chemical Properties Color and Form:red pwdr. Molecular Weight:159.69 Melting Point:1565° Boiling Point:no data Vapor Pressure:no data Specific Gravity:no data Odor:none Solubility in Water:insoluble SECTION 10: Stability and Reactivity Stability:air and moisture stable solid Hazardous Polymerization:no hazardous polymerization Conditions to Avoid:none Incompatibility:Powdered aluminum, hydrazine, ethylene oxide, calcium hypochlorite and magnesium Decomposition Products:none SECTION 11: Toxicological Information RTECS Data:No specific information available in the RTECS files. Carcinogenic Effects:No data available Mutagenic Effects:No data available Tetratogenic Effects:No data available SECTION 12: Ecological Information Ecological Information:No information available SECTION 13: Disposal Considerations Disposal:Dispose of according to local, state and federal regulations. SECTION 14: Transportation Shipping Name (CFR):Non-hazardous Hazard Class (CFR):NA Additional Hazard Class (CFR):NA Packaging Group (CFR):NA UN ID Number (CFR):NA Shipping Name (IATA):Non-hazardous Hazard Class (IATA):NA Additional Hazard Class (IATA):NA Packaging Group (IATA):NA UN ID Number (IATA):NA SECTION 15: Regulatory Information TSCA:Listed in the TSCA inventory. SARA (Title 313):Title compound not listed. Second Ingredient:none SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
|
| 危害码 (欧洲) | Xi: Irritant; |
|---|---|
| 风险声明 (欧洲) | R36/37/38 |
| 安全声明 (欧洲) | S26 |
| WGK德国 | - |
| RTECS号 | NO7400000 |
| 海关编码 | 2601111000 |
| 海关编码 | 2601111000 |
|---|