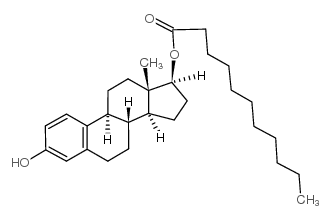
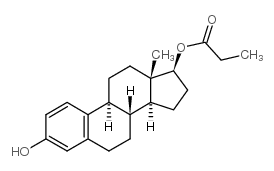
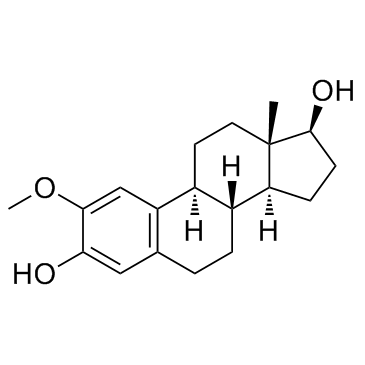
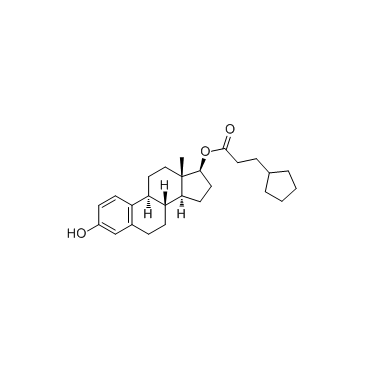
毒理学数据:
急性毒性:
主要的刺激性影响:
在皮肤上面:刺激皮肤和黏膜
在眼睛上面:刺激的影响。
致敏作用:没有已知的敏化影响。
生态学数据:
总括注解
水危害级别3(德国规例)(通过名单进行自我评估)该物质对水有极其危害的。
即使是小量不要让该产品接触地下水、水道或污水系统。
即使是极其小量的产品渗入地下也会对饮用水造成危险
若无政府许可,勿将材料排入周围环境。
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
KG2975000
-
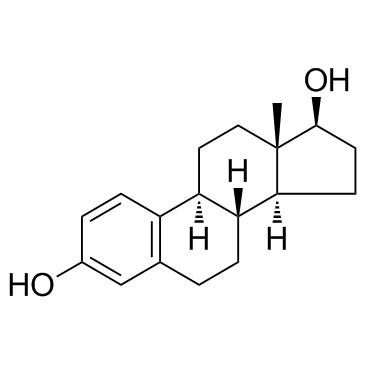
CHEMICAL NAME :
-
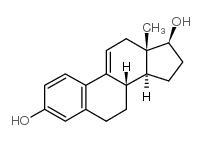
Estradiol
-
CAS REGISTRY NUMBER :
-
50-28-2
-
LAST UPDATED :
-
199710
-
DATA ITEMS CITED :
-
137
-
MOLECULAR FORMULA :
-
C18-H24-O2
-
MOLECULAR WEIGHT :
-
272.42
-
WISWESSER LINE NOTATION :
-
L E5 B666TTT&J E1 FQ OQ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>300 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4200 ug/kg/12W-I
-
TOXIC EFFECTS :
-
Blood - changes in erythrocyte (RBC) count Nutritional and Gross Metabolic - weight loss or decreased weight gain Related to Chronic Data - changes in uterine weight
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1400 mg/kg/13W-I
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Endocrine - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Implant
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
100 mg/kg/52W-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Skin and Appendages - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
84 mg/kg/20W-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Reproductive - Tumorigenic effects - uterine tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
7 mg/kg/12W-I
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Reproductive - Tumorigenic effects - uterine tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Implant
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
1200 ug/kg
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Tumorigenic - tumors at site of application
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Implant
-
SPECIES OBSERVED :
-
Rodent - hamster
-
DOSE/DURATION :
-
200 mg/kg
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Kidney, Ureter, Bladder - Kidney tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Implant
-
SPECIES OBSERVED :
-
Rodent - hamster
-
DOSE/DURATION :
-
360 mg/kg/15W-I
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Kidney, Ureter, Bladder - Kidney tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Implant
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
2400 ug/kg
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Reproductive - Tumorigenic effects - uterine tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
58 mg/kg/82W-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Skin and Appendages - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Implant
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
40 mg/kg
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Reproductive - Tumorigenic effects - uterine tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Implant
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
100 mg/kg
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Reproductive - Tumorigenic effects - uterine tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
44 mg/kg/52W-I
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Skin and Appendages - tumors Tumorigenic - tumor types after systemic administration not seen spontaneously
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Implant
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
62500 ug/kg/36W-I
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Endocrine - tumors Skin and Appendages - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Implant
-
SPECIES OBSERVED :
-
Rodent - hamster
-
DOSE/DURATION :
-
160 mg/kg
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Kidney, Ureter, Bladder - tumors Lungs, Thorax, or Respiration - bronchiogenic carcinoma
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Implant
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
30 mg/kg
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Skin and Appendages - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Implant
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
34 mg/kg
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Skin and Appendages - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
4400 ug/kg
-
SEX/DURATION :
-
female 31 week(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1 gm/kg
-
SEX/DURATION :
-
female 4-8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
750 ug/kg
-
SEX/DURATION :
-
female 3 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
875 ug/kg
-
SEX/DURATION :
-
female 7 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
4195 ng/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
1280 ng/kg
-
SEX/DURATION :
-
male 8 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - other effects on male Endocrine - changes in luteinizing hormone
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
2400 ng/kg
-
SEX/DURATION :
-
female 3 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
205 ug/kg
-
SEX/DURATION :
-
male 5 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - testes, epididymis, sperm duct
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
20 ug/kg
-
SEX/DURATION :
-
female 4 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
14400 ng/kg
-
SEX/DURATION :
-
female 5-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
10500 ng/kg
-
SEX/DURATION :
-
female 7 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
6250 ug/kg
-
SEX/DURATION :
-
female 16-20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
15300 ng/kg
-
SEX/DURATION :
-
female 1-9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
500 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - menstrual cycle changes or disorders
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
10 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
2 ug/kg
-
SEX/DURATION :
-
female 4 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
60 mg/kg
-
SEX/DURATION :
-
female 15-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
60 mg/kg
-
SEX/DURATION :
-
female 19-20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - endocrine system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
1800 mg/kg
-
SEX/DURATION :
-
female 15-20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - ovaries, fallopian tubes
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
6720 ng/kg
-
SEX/DURATION :
-
male 14 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count) Reproductive - Paternal Effects - testes, epididymis, sperm duct Reproductive - Paternal Effects - prostate, seminal vesicle, Cowper's gland, accessory glands
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
70 ug/kg
-
SEX/DURATION :
-
female 14 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - menstrual cycle changes or disorders Reproductive - Maternal Effects - other effects
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
4 ug/kg
-
SEX/DURATION :
-
female 14-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
1600 ug/kg
-
SEX/DURATION :
-
male 3 week(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count) Reproductive - Paternal Effects - testes, epididymis, sperm duct
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Implant
-
DOSE :
-
3600 ng/kg
-
SEX/DURATION :
-
male 90 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - prostate, seminal vesicle, Cowper's gland, accessory glands
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Implant
-
DOSE :
-
437 ug/kg
-
SEX/DURATION :
-
male 91 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count) Reproductive - Paternal Effects - testes, epididymis, sperm duct
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Implant
-
DOSE :
-
262 ug/kg
-
SEX/DURATION :
-
male 91 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count) Reproductive - Paternal Effects - prostate, seminal vesicle, Cowper's gland, accessory glands Reproductive - Fertility - male fertility index (e.g. # males impregnating females per # males exposed to fertile nonpregnant females)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
5 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intrauterine
-
DOSE :
-
25 ng/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
219 mg/kg
-
SEX/DURATION :
-
female 52 week(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - ovaries, fallopian tubes Reproductive - Maternal Effects - uterus, cervix, vagina
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
667 ng/kg
-
SEX/DURATION :
-
female 3 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
4 mg/kg
-
SEX/DURATION :
-
female 5 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - menstrual cycle changes or disorders
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
10 mg/kg
-
SEX/DURATION :
-
male 5 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - testes, epididymis, sperm duct Reproductive - Paternal Effects - prostate, seminal vesicle, Cowper's gland, accessory glands Reproductive - Fertility - male fertility index (e.g. # males impregnating females per # males exposed to fertile nonpregnant females)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
1 mg/kg
-
SEX/DURATION :
-
male 5 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
20 mg/kg
-
SEX/DURATION :
-
female 19 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - delayed effects
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
12 ug/kg
-
SEX/DURATION :
-
female 1-3 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
14400 ng/kg
-
SEX/DURATION :
-
female 4-6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
204 ng/kg
-
SEX/DURATION :
-
female 3 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
2 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
9600 ug/kg
-
SEX/DURATION :
-
female 4-6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
4800 ug/kg
-
SEX/DURATION :
-
female 4-6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
4 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Implant
-
DOSE :
-
1720 ug/kg
-
SEX/DURATION :
-
female 16-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - parturition Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
10 mg/kg
-
SEX/DURATION :
-
female 1-6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated)
-
TYPE OF TEST :
-
TCLo - Lowest published toxic concentration
-
ROUTE OF EXPOSURE :
-
Inhalation
-
DOSE :
-
30 ug/kg/30M
-
SEX/DURATION :
-
male 60 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count) Reproductive - Paternal Effects - testes, epididymis, sperm duct
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
60 ug/kg
-
SEX/DURATION :
-
male 8 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - testes, epididymis, sperm duct Reproductive - Paternal Effects - prostate, seminal vesicle, Cowper's gland, accessory glands
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
50 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
90 ug/kg
-
SEX/DURATION :
-
female 6-11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
45 mg/kg
-
SEX/DURATION :
-
female 1-3 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
45 ug/kg
-
SEX/DURATION :
-
female 5-7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
30 ug/kg
-
SEX/DURATION :
-
female 18-20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth) Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
5 ug/kg
-
SEX/DURATION :
-
female 1-3 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
190 ug/kg
-
SEX/DURATION :
-
female 1-19 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
7692 ng/kg
-
SEX/DURATION :
-
female 9-10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
90 ug/kg
-
SEX/DURATION :
-
female 1-9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
900 ug/kg
-
SEX/DURATION :
-
female 1-9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Implant
-
DOSE :
-
160 mg/kg
-
SEX/DURATION :
-
male 50 week(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - testes, epididymis, sperm duct
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
15 mg/kg
-
SEX/DURATION :
-
male 15 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - testes, epididymis, sperm duct Reproductive - Paternal Effects - prostate, seminal vesicle, Cowper's gland, accessory glands
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
14 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
126 ug/kg
-
SEX/DURATION :
-
female 48 week(s) pre-mating female 1-28 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - menstrual cycle changes or disorders Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Implant
-
DOSE :
-
900 ug/kg
-
SEX/DURATION :
-
male 26 week(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - testes, epididymis, sperm duct
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Implant
-
DOSE :
-
1 mg/kg
-
SEX/DURATION :
-
lactating female 26-47 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - menstrual cycle changes or disorders
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Implant
-
DOSE :
-
147 mg/kg
-
SEX/DURATION :
-
male 82 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - testes, epididymis, sperm duct Reproductive - Paternal Effects - other effects on male
-
TYPE OF TEST :
-
Morphological transformation
-
TYPE OF TEST :
-
Mutation test systems - not otherwise specified
-
TYPE OF TEST :
-
Unscheduled DNA synthesis
-
TYPE OF TEST :
-
Unscheduled DNA synthesis
-
TYPE OF TEST :
-
Unscheduled DNA synthesis
-
TYPE OF TEST :
-
Mutation test systems - not otherwise specified
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TYPE OF TEST :
-
Micronucleus test
-
TYPE OF TEST :
-
Unscheduled DNA synthesis
-
TYPE OF TEST :
-
DNA inhibition
-
TYPE OF TEST :
-
Sister chromatid exchange
-
TYPE OF TEST :
-
Sister chromatid exchange
-
TYPE OF TEST :
-
Sperm Morphology
-
TYPE OF TEST :
-
DNA damage
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TYPE OF TEST :
-
Unscheduled DNA synthesis
MUTATION DATA
-
TEST SYSTEM :
-
Bird - chicken
-
DOSE/DURATION :
-
25 mg/kg
-
REFERENCE :
-
BBRCA9 Biochemical and Biophysical Research Communications. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 112,425,1983 *** REVIEWS *** IARC Cancer Review:Animal Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 21,279,1979 IARC Cancer Review:Animal Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 6,99,1974 TOXICOLOGY REVIEW CLPTAT Clinical Pharmacology and Therapeutics (St. Louis). (C.V. Mosby Co., 11830 Westline Industrial Dr., St. Louis, MO 63146) V.1- 1960- Volume(issue)/page/year: 5,480,1964 TOXICOLOGY REVIEW ADVPA3 Advances in Pharmacology. (New York, NY) V.1-6, 1962-68. For publisher information, see AVPCAQ. Volume(issue)/page/year: 4,263,1966 TOXICOLOGY REVIEW CANCAR Cancer (Philadelphia). (Lippincott/Harper, Journal Fulfillment Dept., 2350 Virginia Ave., Hagerstown, MD 21740) V.1- 1948- Volume(issue)/page/year: 40,1825,1977 TOXICOLOGY REVIEW ACRSAJ Advances in Cancer Research. (Academic Press, Inc., 465 S. Lincoln Dr., Troy, MO 63379) V.1- 1953- Volume(issue)/page/year: 1,173,1953 TOXICOLOGY REVIEW CLECAP Clinical Endocrinology (Oxford). (Blackwell Scientific Pub. Ltd., POB 88, Oxford, UK) V.1- 1972- Volume(issue)/page/year: 4,551,1975 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOHS - National Occupational Hazard Survey (1974) NOHS Hazard Code - 80407 No. of Facilities: 68 (estimated) No. of Industries: 1 No. of Occupations: 6 No. of Employees: 2770 (estimated) NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - 80407 No. of Facilities: 151 (estimated) No. of Industries: 4 No. of Occupations: 5 No. of Employees: 9083 (estimated) No. of Female Employees: 8217 (estimated)
|