722544-51-6
| 中文名 | 5-[[7-[3-[乙基(2-羟基乙基)氨基]丙氧基]-4-喹唑啉]氨基]-N-(3-氟苯基)-1H-吡唑-3-乙酰胺 |
|---|---|
| 英文名 | 2-{3-[(7-[3-[ethyl(2-hydroxyethyl)amino]propoxy]-quinazolin-4-yl)amino]-1H-pyrazol-5-yl}-N-(3-fluorophenyl)acetamide |
| 中文别名 | 多西他赛 |
| 英文别名 |
AZD1152-HQPA
INH 34 AZD-1152-HQPA 2-{3-[(7-{3-[Ethyl(2-hydroxyethyl)amino]propoxy}-4-quinazolinyl)amino]-1H-pyrazol-5-yl}-N-(3-fluorophenyl)acetamide Barasertib 2-{3-[(7-{3-[ethyl(2-hydroxyethyl)amino]propoxy}quinazolin-4-yl)amino]-1H-pyrazol-5-yl}-N-(3-fluorophenyl)acetamide AZD1152 HPQA |
| 描述 | AZD1152-HQPA 是一种高度选择性的 Aurora B 抑制剂,IC50 值为 0.37 nM,对 Aurora B 的选择性是 Aurora A 的 3700 倍。 |
|---|---|
| 相关类别 | |
| 靶点 |
Aurora B:0.37 nM (IC50) |
| 体外研究 | 与极光A相比,AZD1152对Aurora B的选择性> 3000倍,其IC50为1.368μM。 AZD1152对50种其他丝氨酸-苏氨酸和酪氨酸激酶(包括FLT3,JAK2和Abl)的活性甚至更低。 AZD1152抑制造血系统恶性细胞如HL-60,NB4,MOLM13,PALL-1,PALL-2,MV4-11,EOL-1,THP-1和K562细胞的增殖,IC50为3-40 nM,显示比另一种极光激酶抑制剂ZM334739的效力高100倍,其IC50为3-30μM。 AZD1152抑制MOLM13和MV4-11细胞的克隆生长,IC50分别为1 nM和2.8 nM,以及新鲜分离的伊马替尼耐药白血病细胞,IC50值为1-3 nM,与骨髓单核细胞相比更为显着IC50值> 10 nM的细胞。 AZD1152诱导4N/8N DNA含量的细胞积累,然后以剂量和时间依赖的方式凋亡[2]。 AZD1152-HQPA处理诱导LNCaP细胞系中的缺陷细胞存活,多倍体和细胞死亡。 AZD1152-HQPA也可降低AR的表达[3]。 |
| 体内研究 | AZD1152(10-150mg/kg /天)以剂量依赖性方式显着抑制多种人实体瘤异种移植物(包括结肠癌,乳腺癌和肺癌)的生长[1]。单独施用AZD1152(25mg/kg)显着抑制MOLM13异种移植物的生长,通过观察具有吞噬细胞浸润的坏死组织证实[2]。 |
| 细胞实验 | 将细胞暴露于各种浓度的AZD1152 24或48小时。通过3H-胸苷摄取(在收获前6小时添加同位素)测量细胞增殖,并且从剂量 - 反应曲线计算诱导50%生长抑制的浓度(IC 50)。通过流式细胞术进行细胞周期分析。通过膜联蛋白V-FITC细胞凋亡检测试剂盒测量细胞凋亡。 |
| 动物实验 | 通过皮下注射100至200μL肿瘤细胞(在与基质胶50:50混合的1×10 6和1×10 7个细胞之间)来建立人肿瘤异种移植物。当肿瘤达到确定的可触知大小(分别为小鼠和大鼠0.2-0.3cm 3和0.5-1cm 3)时,将动物随机分成治疗组(每组n = 8-11)。 AZD1152在Tris缓冲液(pH 9)中制备,并按照推注注射(静脉注射或腹腔注射)或连续48小时输注,通过sc植入的渗透微型泵(按顺序植入两个24小时泵)按照制造商的说明。用卡尺每周测量肿瘤最多三次,计算肿瘤体积,并使用每组的几何平均值对时间绘制数据。计算肿瘤体积和肿瘤生长抑制。使用Student's单尾t检验进行肿瘤体积变化的统计分析(P值<0.05被认为是统计学上显着的)。 |
| 参考文献 |
| 密度 | 1.4±0.1 g/cm3 |
|---|---|
| 沸点 | 796.7±60.0 °C at 760 mmHg |
| 分子式 | C26H30FN7O3 |
| 分子量 | 507.560 |
| 闪点 | 435.6±32.9 °C |
| 精确质量 | 507.239410 |
| PSA | 128.29000 |
| LogP | 2.82 |
| 外观性状 | white to beige |
| 蒸汽压 | 0.0±2.9 mmHg at 25°C |
| 折射率 | 1.677 |
| 储存条件 | 2-8°C |
| 水溶解性 | DMSO: >15mg/mL |
|
SECTION 1: Identification of the substance/mixture and of the company/undertaking Product identifiers Product name: AZD1152-HQPA : SML0268 REACH No.: A registration number is not available for this substance as the substance or its uses are exempted from registration, the annual tonnage does not require a registration or the registration is envisaged for a later
registration deadline. CAS-No.: 722544-51-6 SECTION 2: Hazards identification Classification of the substance or mixture Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008. This substance is not classified as dangerous according to Directive 67/548/EEC. Label elements This substance is not classified as dangerous according to Directive 67/548/EEC. Other hazards - none SECTION 3: Composition/information on ingredients Substances Synonyms: 3-[[7-[3-[Ethyl(2-hydroxyethyl)amino]propoxy]-4-quinazolinyl]amino]-N-(3- fluorophenyl)-1H-pyrazole-5-acetamide Formula: C26H30FN7O3 Molecular Weight: 507,56 g/mol CAS-No.: 722544-51-6 No components need to be disclosed according to the applicable regulations. SECTION 4: First aid measures Description of first aid measures General advice Consult a physician. Show this safety data sheet to the doctor in attendance. If inhaled If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician. In case of skin contact Wash off with soap and plenty of water. Consult a physician. In case of eye contact Flush eyes with water as a precaution. If swallowed Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. Most important symptoms and effects, both acute and delayed The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in section 11 Indication of any immediate medical attention and special treatment needed no data available SECTION 5: Firefighting measures Extinguishing media Suitable extinguishing media Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide. Special hazards arising from the substance or mixture Carbon oxides, nitrogen oxides (NOx), Hydrogen fluoride Advice for firefighters Wear self contained breathing apparatus for fire fighting if necessary. Further information no data available SECTION 6: Accidental release measures Personal precautions, protective equipment and emergency procedures Use personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Avoid breathing dust. For personal protection see section 8. Environmental precautions Do not let product enter drains. Methods and materials for containment and cleaning up Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed containers for disposal. Reference to other sections For disposal see section 13. SECTION 7: Handling and storage Precautions for safe handling Avoid formation of dust and aerosols. Provide appropriate exhaust ventilation at places where dust is formed. For precautions see section 2.2. Conditions for safe storage, including any incompatibilities Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Recommended storage temperature: 2 - 8 °C Specific end use(s) A part from the uses mentioned in section 1.2 no other specific uses are stipulated SECTION 8: Exposure controls/personal protection Control parameters Components with workplace control parameters Exposure controls Appropriate engineering controls Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday. Personal protective equipment Eye/face protection Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU). Skin protection Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it. Body Protection Choose body protection in relation to its type, to the concentration and amount of dangerous substances, and to the specific work-place., The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Respiratory protection Respiratory protection is not required. Where protection from nuisance levels of dusts are desired, use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and approved under appropriate government standards such as NIOSH (US) or CEN (EU). Control of environmental exposure Do not let product enter drains. SECTION 9: Physical and chemical properties Information on basic physical and chemical properties a) AppearanceForm: solid b) Odourno data available c) Odour Thresholdno data available d) pHno data available e) Melting point/freezingno data available point f) Initial boiling point and no data available boiling range g) Flash pointno data available h) Evapouration rateno data available i) Flammability (solid, gas) no data available j) Upper/lowerno data available flammability or explosive limits k) Vapour pressureno data available l) Vapour densityno data available m) Relative densityno data available n) Water solubilityno data available o) Partition coefficient: n- no data available octanol/water p) Auto-ignitionno data available temperature q) Decompositionno data available temperature r) Viscosityno data available s) Explosive propertiesno data available t) Oxidizing propertiesno data available Other safety information no data available SECTION 10: Stability and reactivity Reactivity no data available Chemical stability Stable under recommended storage conditions. Possibility of hazardous reactions no data available Conditions to avoid no data available Incompatible materials Strong oxidizing agents Hazardous decomposition products Other decomposition products - no data available In the event of fire: see section 5 SECTION 11: Toxicological information Information on toxicological effects Acute toxicity no data available Skin corrosion/irritation no data available Serious eye damage/eye irritation no data available Respiratory or skin sensitisation no data available Germ cell mutagenicity no data available Carcinogenicity IARC:No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC. Reproductive toxicity no data available Specific target organ toxicity - single exposure no data available Specific target organ toxicity - repeated exposure no data available Aspiration hazard no data available Additional Information RTECS: Not available To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly investigated. SECTION 12: Ecological information Toxicity no data available Persistence and degradability no data available Bioaccumulative potential no data available Mobility in soil no data available Results of PBT and vPvB assessment PBT/vPvB assessment not available as chemical safety assessment not required/not conducted Other adverse effects no data available SECTION 13: Disposal considerations Waste treatment methods Product Offer surplus and non-recyclable solutions to a licensed disposal company. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. Contaminated packaging Dispose of as unused product. SECTION 14: Transport information UN number ADR/RID: -IMDG: -IATA: - UN proper shipping name ADR/RID: Not dangerous goods IMDG: Not dangerous goods IATA:Not dangerous goods Transport hazard class(es) ADR/RID: -IMDG: -IATA: - Packaging group ADR/RID: -IMDG: -IATA: - Environmental hazards ADR/RID: noIMDG Marine pollutant: noIATA: no Special precautions for user no data available SECTION 15: Regulatory information This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006. Safety, health and environmental regulations/legislation specific for the substance or mixture no data available Chemical Safety Assessment SECTION 16 - ADDITIONAL INFORMATION N/A |
| 危险品运输编码 | NONH for all modes of transport |
|---|
|
~82% 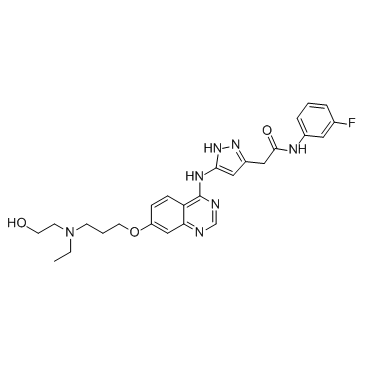
722544-51-6 |
| 文献:ASTRAZENECA AB; ASTRAZENECA UK LIMITED Patent: WO2007/132227 A1, 2007 ; Location in patent: Page/Page column 26; 30 ; WO 2007/132227 A1 |
|
~% 
722544-51-6 |
| 文献:Journal of Medicinal Chemistry, , vol. 50, # 9 p. 2213 - 2224 |
|
~% 
722544-51-6 |
| 文献:Journal of Medicinal Chemistry, , vol. 50, # 9 p. 2213 - 2224 |
|
~% 
722544-51-6 |
| 文献:Journal of Medicinal Chemistry, , vol. 50, # 9 p. 2213 - 2224 |
|
~% 
722544-51-6 |
| 文献:Journal of Medicinal Chemistry, , vol. 50, # 9 p. 2213 - 2224 |
|
~% 
722544-51-6 |
| 文献:Journal of Medicinal Chemistry, , vol. 50, # 9 p. 2213 - 2224 |
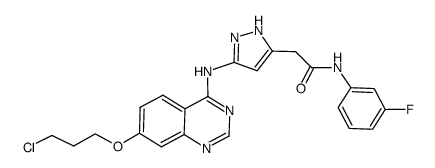
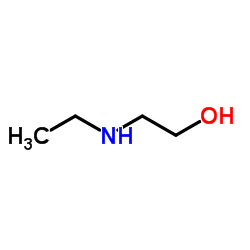
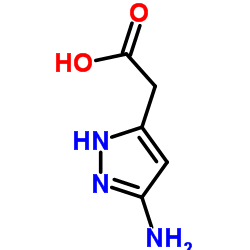
| 上游产品 7 | |
|---|---|
| 下游产品 1 | |


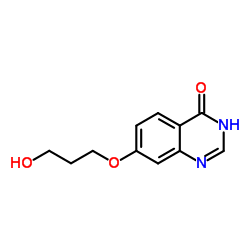
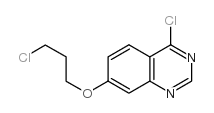
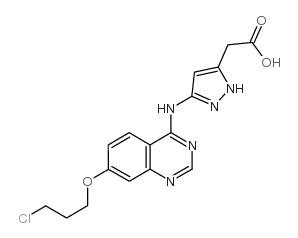
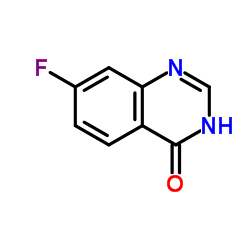
![3-[[7-[3-[乙基[2-(磷酰氧基)乙基]氨基]丙氧基]-4-喹唑啉基]氨基]-N-(3-氟苯基)-1H-吡唑-5-乙酰胺结构式](https://image.chemsrc.com/caspic/416/957881-03-7.png)