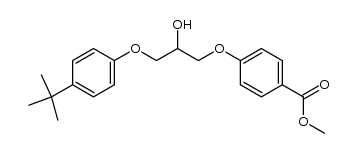
56488-59-6
| 中文名 | 特丁贝罗 |
|---|---|
| 英文名 | 4-[3-(4-tert-butylphenoxy)-2-hydroxypropoxy]benzoic acid |
| 英文别名 |
4'-(3-(4'-t-Butylphenoxy)-2-hydroxypropoxy)benzoic acid
4-(3-(4-(1,1-Dimethylethyl)phenoxy)-2-hydroxypropoxy)benzoic acid K-11,267 Terbufibrolum [INN-Latin] BENZOIC ACID,p-(3-(p-tert-BUTYLPHENOXY)-2-HYDROXYPROPOXY) Terbufibrol p-(3-(p-tert-Butylphenoxy)-2-hydroxypropoxy)benzoic acid Terbufibrolum Benzoic acid,4-(3-(4-(1,1-dimethylethyl)phenoxy)-2-hydroxypropoxy) |
| 描述 | Terbufibrol 在正常和高胆固醇血症雄性大鼠中,高效降低血清总胆固醇 (TC) 水平。 |
|---|---|
| 相关类别 | |
| 体内研究 | 特比布罗(20-200 mg/kg)在所有3种饮食中均可降低动物血清TC,但胆固醇降低的程度依次为胆固醇(HC)>高蛋白和脂肪饮食(HPF)> N(最大值。减少:分别为154%,80%和70%)。使用HPF饮食时,氯贝特(200 mg/kg)使TC降低最多28%,使用N饮食降低13%,但对HC饮食无效。消胆胺(400mg/kg /天)在HPF喂养的动物中无活性,但在HC喂养的动物中TC降低45%。使用HPF饮食烟酸(200 mg/kg)无效。替比福布降低喂食HPF饮食的动物的HDL,HDL-TC,LDL,LDL-TC和LDL-TC/PL。随着剂量增加,LDL-TC出现更大的降低。氯贝特(200 mg/kg)的主要作用是降低HDL和HDL-TC并增加LDL [1]。 |
| 动物实验 | 狒狒[1] 18只狒狒(9只雄性,9只雌性)全程给予N-饮食,分成3组,每组6只,用特比布罗治疗8周,如下:第1组:对照;第2组:每天50毫克/千克;第3组:每天200毫克/千克。 |
| 参考文献 |
| 密度 | 1.179g/cm3 |
|---|---|
| 沸点 | 524.1ºC at 760mmHg |
| 分子式 | C20H24O5 |
| 分子量 | 344.40200 |
| 闪点 | 183.4ºC |
| 精确质量 | 344.16200 |
| PSA | 75.99000 |
| LogP | 3.50100 |
| 折射率 | 1.566 |
| 储存条件 | 库房通风低温干燥 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
56488-59-6 |
| 文献:Klinge Pharma GmbH and Co Patent: DE2460689 , 1976 ; Chem.Abstr., 1979 , vol. 90, # 22564 |
|
~% 
56488-59-6 |
| 文献:Klinge Pharma GmbH and Co Patent: DE2460689 , 1976 ; Chem.Abstr., 1979 , vol. 90, # 22564 |
|
~% 
56488-59-6 |
| 文献:Klinge Pharma GmbH and Co Patent: DE2460689 , 1976 ; Chem.Abstr., 1979 , vol. 90, # 22564 |
| 上游产品 3 | |
|---|---|
| 下游产品 0 | |