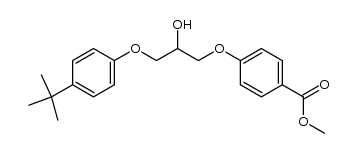
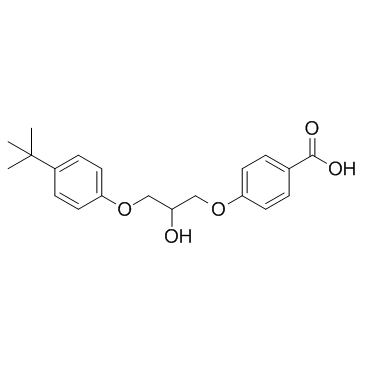
56488-59-6
| Name | 4-[3-(4-tert-butylphenoxy)-2-hydroxypropoxy]benzoic acid |
|---|---|
| Synonyms |
4'-(3-(4'-t-Butylphenoxy)-2-hydroxypropoxy)benzoic acid
4-(3-(4-(1,1-Dimethylethyl)phenoxy)-2-hydroxypropoxy)benzoic acid K-11,267 Terbufibrolum [INN-Latin] BENZOIC ACID,p-(3-(p-tert-BUTYLPHENOXY)-2-HYDROXYPROPOXY) Terbufibrol p-(3-(p-tert-Butylphenoxy)-2-hydroxypropoxy)benzoic acid Terbufibrolum Benzoic acid,4-(3-(4-(1,1-dimethylethyl)phenoxy)-2-hydroxypropoxy) |
| Description | Terbufibrol has been shown highly active in reducing serum total cholesterol (TC) levels in the normal and hypercholesterolemic male rat. |
|---|---|
| Related Catalog | |
| In Vivo | Terbufibrol (20-200 mg/kg) is active in lowering serum TC in animals on all 3 diets, but the extent of cholesterol reduction varies in the order cholesterol (HC)>high protein and fat diet (HPF)>N (max. reductions: 154, 80, and 70%, respectively). Clofibrate (200 mg/kg) decreases TC by a maximum of 28% with the HPF-diet and 13% with the N-diet but is inactive with the HC-diet. Cholestyramine (400 mg/kg/day) is inactive in HPF-fed animals but reduces TC in HC-fed animals by 45%. With the HPF-diet nicotinic acid (200 mg/kg) is inactive. Terbufibrol lowers HDL, HDL-TC, LDL, LDL-TC and LDL-TC/PL in animals fed HPF-diet. A greater decrease of LDL-TC occurres with increasing dose. The main effect of Clofibrate (200 mg/kg) is to reduce HDL and HDL-TC and to increase LDL[1]. |
| Animal Admin | Baboon[1] Eighteen baboons (9 male, 9 female) are given the N-diet throughout, divided into 3 groups of 6 animals, and treated with Terbufibrol for 8 weeks, as follows: Group 1: control; Group 2: 50 mg/kg per day; Group 3: 200 mg/kg per day. |
| References |
| Density | 1.179g/cm3 |
|---|---|
| Boiling Point | 524.1ºC at 760mmHg |
| Molecular Formula | C20H24O5 |
| Molecular Weight | 344.40200 |
| Flash Point | 183.4ºC |
| Exact Mass | 344.16200 |
| PSA | 75.99000 |
| LogP | 3.50100 |
| Index of Refraction | 1.566 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
56488-59-6 |
| Literature: Klinge Pharma GmbH and Co Patent: DE2460689 , 1976 ; Chem.Abstr., 1979 , vol. 90, # 22564 |
|
~% 
56488-59-6 |
| Literature: Klinge Pharma GmbH and Co Patent: DE2460689 , 1976 ; Chem.Abstr., 1979 , vol. 90, # 22564 |
|
~% 
56488-59-6 |
| Literature: Klinge Pharma GmbH and Co Patent: DE2460689 , 1976 ; Chem.Abstr., 1979 , vol. 90, # 22564 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |