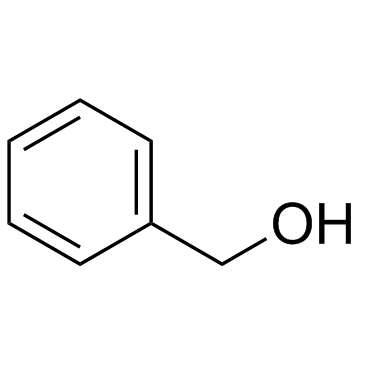
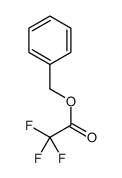
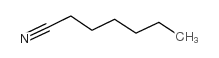
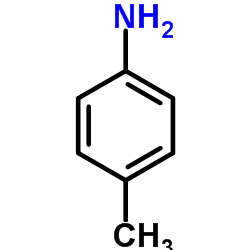
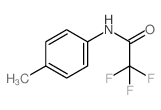
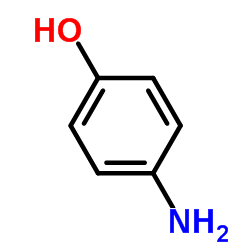
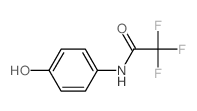
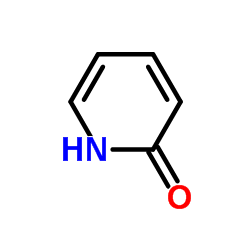
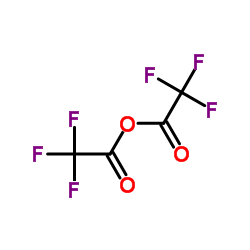
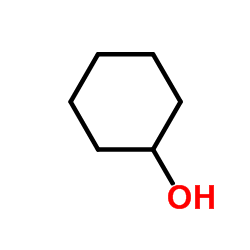
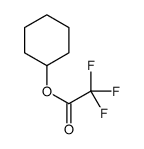
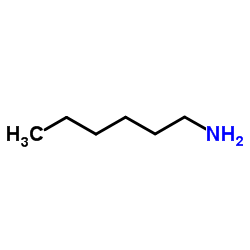
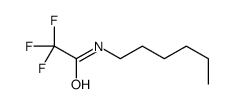
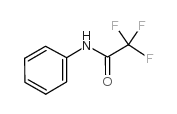
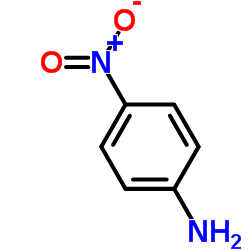
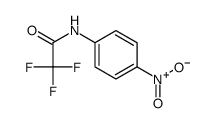
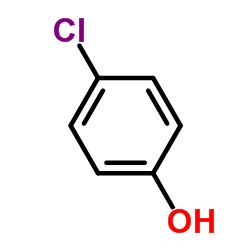
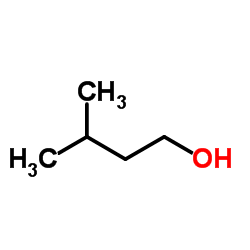
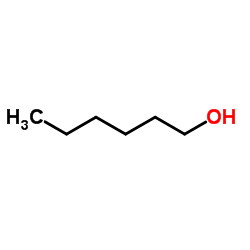
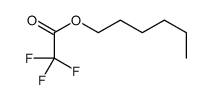
|
~96% |
|
~98% |
|
~93% |
|
~90% |
|
~99% |
|
~88% |
|
~96% |
|
~92% |
|
~91% |
|
~93% |
|
~92% |
|
~91% |
|
~96% |