Organic Letters
2018-04-10
Nitrate-promoted Selective C–H Fluorination of Benzamides and Benzeneacetamides
Xing-Qian Ning, Shao-Jie Lou, Yang-Jie Mao, Zhen-Yuan Xu, Dan-Qian Xu
Index: 10.1021/acs.orglett.8b00793
Full Text: HTML
Abstract
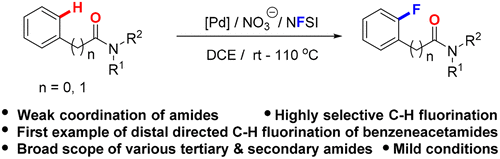
A versatile and site-selective nitrate-promoted C–H bond fluorination using various weak coordinating amides as intrinsic directing groups was developed. Diverse tertiary and secondary amides underwent selective aromatic C–H bond fluorination, which features broad substrate scope, good regioselectivity, and mild conditions. Moreover, the late-stage C–H bond fluorination of the challenging benzeneacetamides via distal directing was reported for the first time.
Latest Articles:
More...
|
Sarinfacetamides A and B, Nitrogenous Diterpenoids with Tric...
2018-04-11 [10.1021/acs.orglett.8b00842] |
|
Studies toward the Synthesis of Iriomoteolide-2a: Constructi...
2018-04-10 [10.1021/acs.orglett.8b00542] |
|
Aroyl Isocyanates as 1,4-Dipoles in a Formal [4 + 1]-Cycload...
2018-04-10 [10.1021/acs.orglett.8b00656] |
|
Controlled Synthesis of C70 Equatorial Multiadducts with Mix...
2018-04-10 [10.1021/acs.orglett.8b00672] |
|
Direct ortho-Selective C–H Functionalization of Carboxybenzy...
2018-04-10 [10.1021/acs.orglett.8b00797] |