Organic Letters
2018-04-10
Aroyl Isocyanates as 1,4-Dipoles in a Formal [4 + 1]-Cycloaddition Approach toward Oxazolone Construction
KaitlynE. Eckert, Brandon L. Ashfeld
Index: 10.1021/acs.orglett.8b00656
Full Text: HTML
Abstract
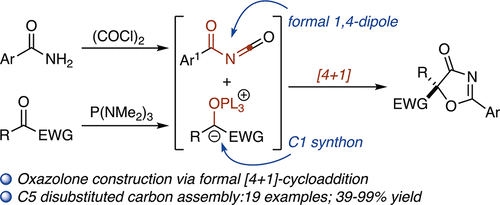
A formal phosphine-mediated [4 + 1]-cycloaddition between a 1,2-dicarbonyl and an aroyl isocyanate to provide oxazolones bearing a disubstituted C5 center is described. By exploiting the carbene-like reactivity of oxyphosphonium enolates as C1 synthons and aroyl isocyanates as formal 1,4-dipoles, oxazolones and spiroooxindole oxazolones are constructed in high yields (39–99%).
Latest Articles:
More...
|
Sarinfacetamides A and B, Nitrogenous Diterpenoids with Tric...
2018-04-11 [10.1021/acs.orglett.8b00842] |
|
Studies toward the Synthesis of Iriomoteolide-2a: Constructi...
2018-04-10 [10.1021/acs.orglett.8b00542] |
|
Controlled Synthesis of C70 Equatorial Multiadducts with Mix...
2018-04-10 [10.1021/acs.orglett.8b00672] |
|
Nitrate-promoted Selective C–H Fluorination of Benzamides an...
2018-04-10 [10.1021/acs.orglett.8b00793] |
|
Direct ortho-Selective C–H Functionalization of Carboxybenzy...
2018-04-10 [10.1021/acs.orglett.8b00797] |