Organic Letters
2018-04-10
Controlled Synthesis of C70 Equatorial Multiadducts with Mixed Addends from an Equatorial Diadduct: Evidence for an Electrophilic Carbanion
Shu-Hui Li, Zong-Jun Li, Wei-Wei Yang, Xiang Gao
Index: 10.1021/acs.orglett.8b00672
Full Text: HTML
Abstract
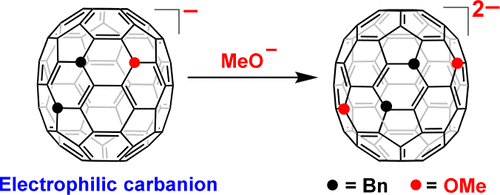
Controlled synthesis of the equatorial tetra-, hexa-, and octaorgano[70]fullerenes with mixed addends was achieved via the reaction of equatorial 7,23-Bn2C70 with MeO– and ArCH2Br. The products were structurally characterized by single crystal X-ray diffraction. The regioselectivity of the reaction was studied by in situ vis–NIR and Fukui function analysis. A surprising electrophilic triorgano[70]fullerene carbanion was shown, and an enhanced fluorescence was observed for the mixed octaadducts.
Latest Articles:
More...
|
Sarinfacetamides A and B, Nitrogenous Diterpenoids with Tric...
2018-04-11 [10.1021/acs.orglett.8b00842] |
|
Studies toward the Synthesis of Iriomoteolide-2a: Constructi...
2018-04-10 [10.1021/acs.orglett.8b00542] |
|
Aroyl Isocyanates as 1,4-Dipoles in a Formal [4 + 1]-Cycload...
2018-04-10 [10.1021/acs.orglett.8b00656] |
|
Nitrate-promoted Selective C–H Fluorination of Benzamides an...
2018-04-10 [10.1021/acs.orglett.8b00793] |
|
Direct ortho-Selective C–H Functionalization of Carboxybenzy...
2018-04-10 [10.1021/acs.orglett.8b00797] |