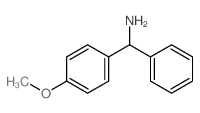
|
~% |
|
~51% |
|
~% |
|
~% |
|
~89% |
|
~% |
|
~% |
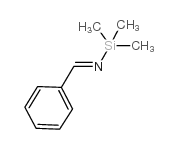
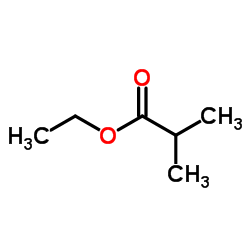
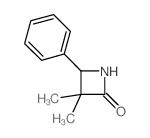
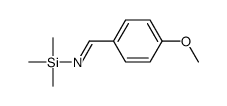
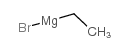
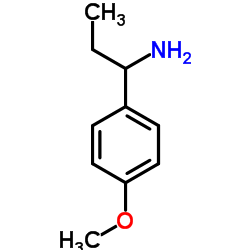

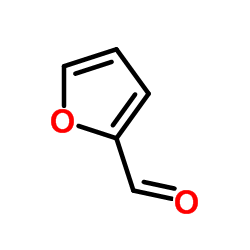
![1-(2-FURAN-2-YL-IMIDAZO[1,2-A]PYRIDIN-3-YLMETHYL)-PIPERIDINE-4-CARBOXYLICACID Structure](https://image.chemsrc.com/caspic/158/83948-38-3.png)