|
~95% |
|
~% |
|
~% |
|
~% |
|
~10% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
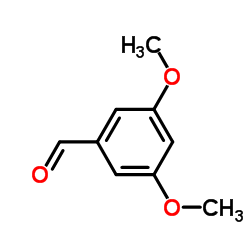
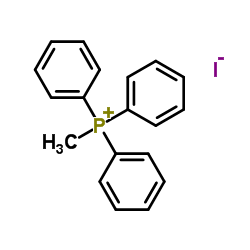
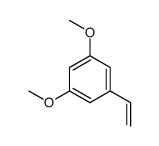
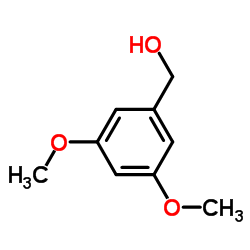
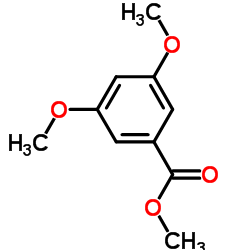
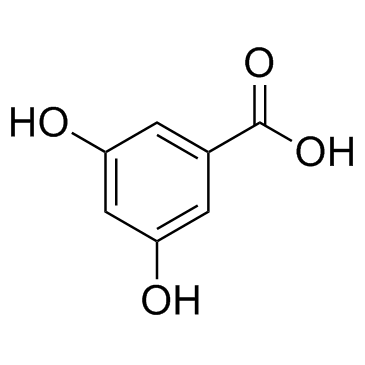

![5,6,7,15-tetrahydroxy-17-methoxy-11-methyl-12-oxabicyclo[12.4.0]octadeca-1(14),2,8,15,17-pentaen-13-one Structure](https://image.chemsrc.com/caspic/267/66018-41-5.png)
![(4S,5S)-4-[(tert-butyldimethylsilyloxy)methyl]-5-[(S)-1-(4-methoxybenzyloxy)but-3-enyl]-2,2-dimethyl-1,3-dioxolane Structure](https://image.chemsrc.com/caspic/038/1403608-16-1.png)
![{(4S,5S)-5-[(S)-1-(4-methoxybenzyloxy)but-3-enyl]-2,2-dimethyl-1,3-dioxolan-4-yl}methanol Structure](https://image.chemsrc.com/caspic/000/1403608-17-2.png)
![(4R,5S)-5-[(S)-1-(4-methoxybenzyloxy)but-3-enyl]-2,2-dimethyl-1,3-dioxolane-4-carbaldehyde Structure](https://image.chemsrc.com/caspic/238/1403608-11-6.png)
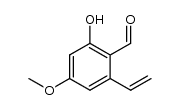
![(4S,5S)-4-[(R,E)-4-tert-butyldiphenylsilyloxypent-1-enyl]-5-[(S)-1-(4-methoxybenzyloxy)but-3-enyl]-2,2-dimethyl-1,3-dioxolane Structure](https://image.chemsrc.com/caspic/462/1403608-18-3.png)
![(R,E)-5-{(4S,5S)-5-[(S)-1-(4-methoxybenzyloxy)but-3-enyl]-2,2-dimethyl-1,3-dioxolan-4-yl}pent-4-en-2-ol Structure](https://image.chemsrc.com/caspic/276/1403608-10-5.png)