| Structure | Name/CAS No. | Articles |
|---|---|---|
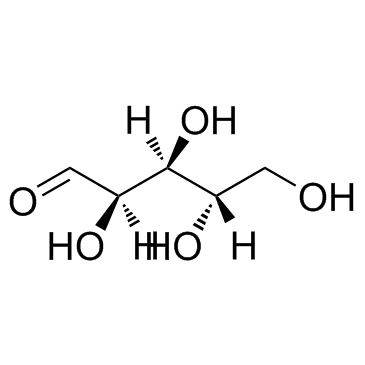 |
L-(+)-Arabinose
CAS:5328-37-0 |
|
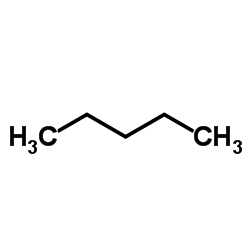 |
Pentane
CAS:109-66-0 |
|
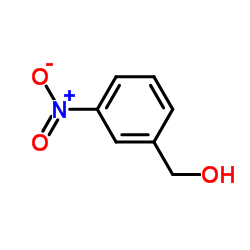 |
3-Nitrobenzenemethanol
CAS:619-25-0 |
|
 |
Chloramphenicol
CAS:56-75-7 |