| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium carbonate
CAS:497-19-8 |
|
 |
Formic Acid
CAS:64-18-6 |
|
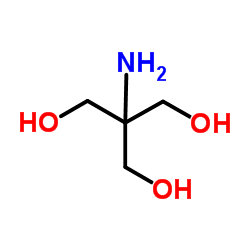 |
Trometamol
CAS:77-86-1 |
|
 |
Methanol
CAS:67-56-1 |
|
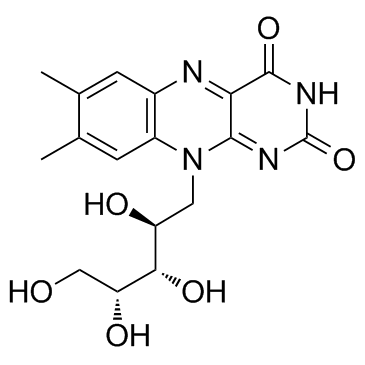 |
Riboflavine
CAS:83-88-5 |
|
 |
L-cysteine
CAS:52-90-4 |
|
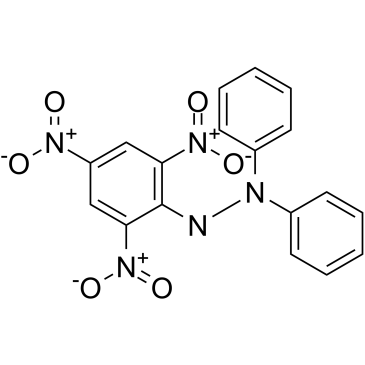 |
DPPH
CAS:1898-66-4 |
|
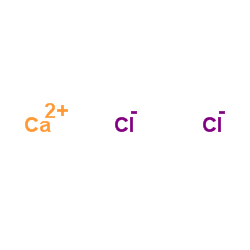 |
Calcium chloride
CAS:10043-52-4 |
|
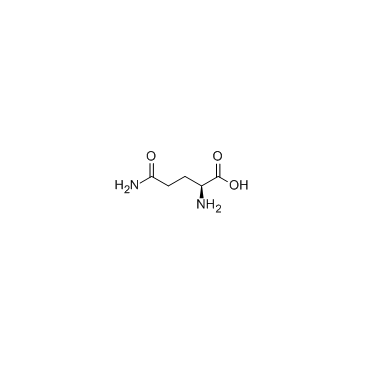 |
L-Glutamine
CAS:56-85-9 |
|
 |
acetic acid
CAS:64-19-7 |