| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium carbonate
CAS:497-19-8 |
|
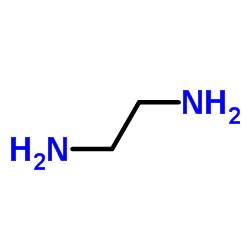 |
1,2-Ethanediamine
CAS:107-15-3 |
|
 |
Hydrochloric acid
CAS:7647-01-0 |
|
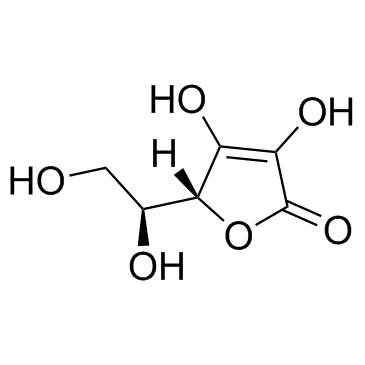 |
Ascorbic acid
CAS:50-81-7 |
|
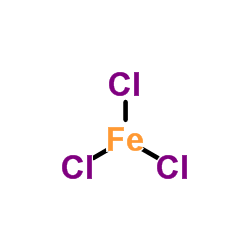 |
Ferric chloride
CAS:7705-08-0 |
|
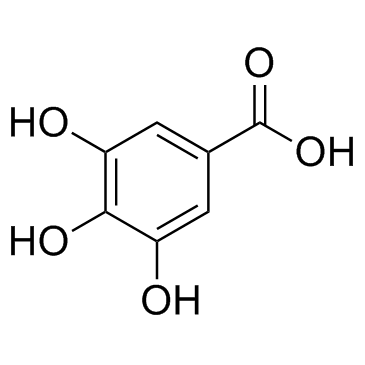 |
Gallic acid
CAS:149-91-7 |
|
 |
L-Ascorbyl 6-palmitate
CAS:137-66-6 |
|
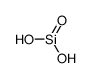 |
silicic acid
CAS:7699-41-4 |
|
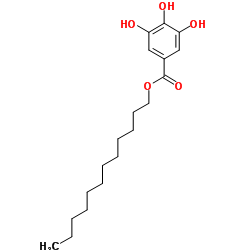 |
Dodecyl gallate
CAS:1166-52-5 |
|
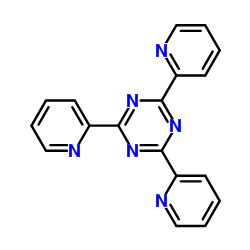 |
2,4,6-Tri(2-pyridinyl)-1,3,5-triazine
CAS:3682-35-7 |