| Structure | Name/CAS No. | Articles |
|---|---|---|
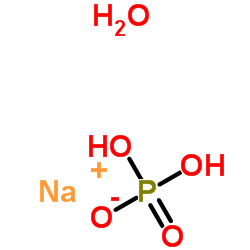 |
Sodium phosphate monobasic monohydrate
CAS:10049-21-5 |
|
 |
Sodium hydroxide
CAS:1310-73-2 |
|
 |
4-aminosalicylic acid
CAS:65-49-6 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Hydrochloric acid
CAS:7647-01-0 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
Salicylic acid
CAS:69-72-7 |
|
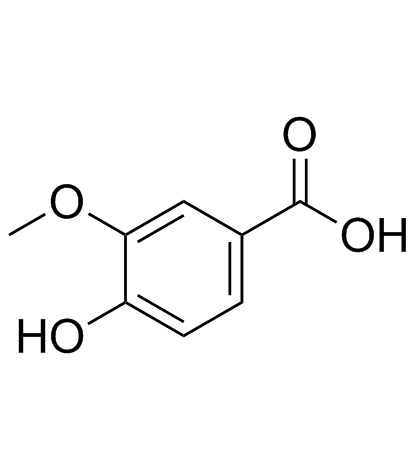 |
Vanillic acid
CAS:121-34-6 |
|
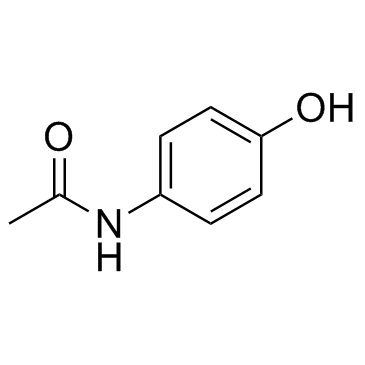 |
4-Acetamidophenol
CAS:103-90-2 |
|
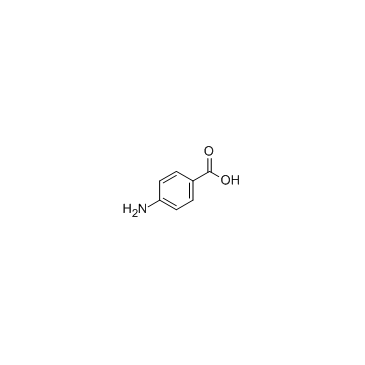 |
4-Aminobenzoic acid
CAS:150-13-0 |