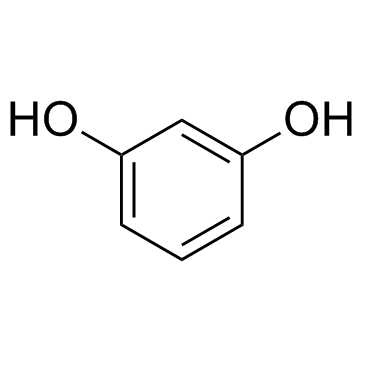
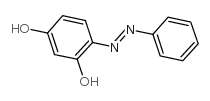
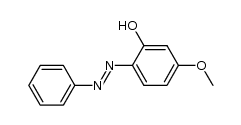
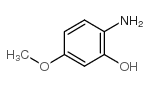
|
~% |
|
~92% |
|
~87% |
|
~81% |
|
~72% |
|
~% |
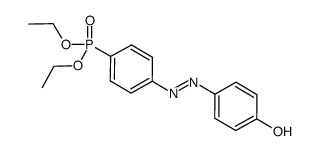
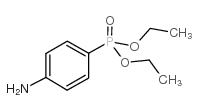
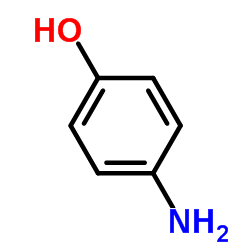
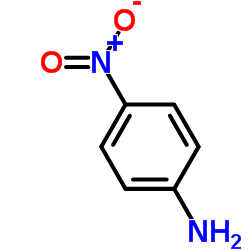

![Phenol,4-[2-(4-nitrophenyl)diazenyl] Structure](https://image.chemsrc.com/caspic/446/1435-60-5.png)

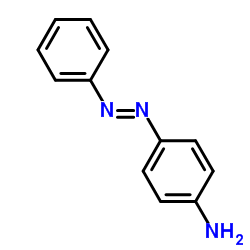
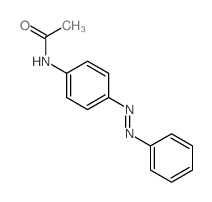
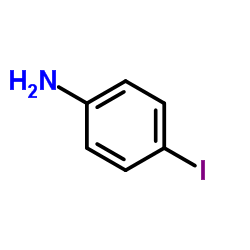
![Phenol,4-[2-(4-iodophenyl)diazenyl] Structure](https://image.chemsrc.com/caspic/374/2703-28-8.png)