| Structure | Name/CAS No. | Articles |
|---|---|---|
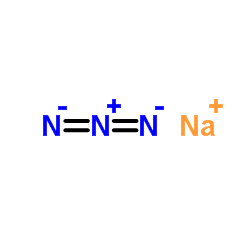 |
Sodium azide
CAS:26628-22-8 |
|
 |
Hydrochloric acid
CAS:7647-01-0 |
|
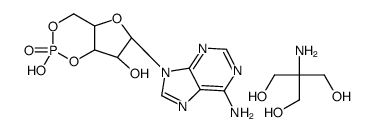 |
ADENOSINE 3':5'-CYCLIC MONOPHOSPHATE TRIS SALT
CAS:102029-77-6 |
|
 |
2-Chlorotrityl Chloride
CAS:42074-68-0 |
|
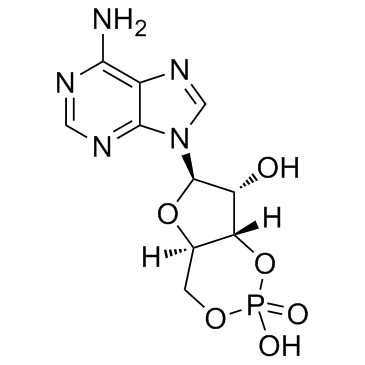 |
Adenosine cyclophosphate
CAS:60-92-4 |