In vitro and in vivo therapeutics of β-thujaplicin on LPS-induced inflammation in macrophages and septic shock in mice.
M-F Shih, L-Y Chen, P-J Tsai, J-Y Cherng
Index: Int. J. Immunopathol. Pharmacol. 25(1) , 39-48, (2012)
Full Text: HTML
Abstract
β-thujaplicin, an active constituent from Chamaecyparis obtusa, has been shown to have acaricidal and antimicrobial effects. Very few studies have focused on the potential of the anti-inflammatory effect of β-thujaplicin. Moreover, its capability of inhibiting inflammatory mediators e.g. TNF-a gene transcription, nitric oxide (NO) and prostaglandin E2, remains unknown. Besides those molecular mechanisms behind the anti-inflammatory effect of β-thujaplicin, solid proof of its effectiveness in vivo has not yet been studied. In our study, in vitro effects of β thujaplicin were verified on RAW 264.7 macrophages which were stimulated by LPS. Indomethacin was used as a positive control. The inducible NO production after stimulation was measured by Griess reagent. PGE2, IL-6 and TNF-α were measured by ELISA methods. Protein expressions of iNOS, COX2, and NF-κB were evaluated by Western blotting. Septic ICR mice were administered 20 mg/kg of LPS and then the mortality rate was monitored. Within the concentration range which was devoid of cytotoxicty, β-thujaplicin exhibited a clear dose-dependent inhibition on LPS-induced NO production. Furthermore, β-thujaplicin inhibited LPS-induced PGE2, IL-6, and TNF-α production as well as iNOS, COX2, and NF- κB protein expression more substantially potent than indomethacin. In agreement with the in vitro study, β-thujaplicin was shown to be effective in vivo for inhibiting LPS-induced NO and TNF-α production and a significant decrease in mortality rate of mice suffering from septic shock was observed. This study demonstrates the potential of β-thujaplicin in treatment of inflammation and sepsis. These effects occur through an efficient blockage of TNF-α and iNOS production. β-thujaplicin efficacy is comparable to that of indomethacin thus it can be a substitution but bear less depletion of PGE2, making this compound very promising in clinical applications. β-thujaplicin, an active constituent from Chamaecyparis obtusa, has been shown to have acaricidal and antimicrobial effects. Very few studies have focused on the potential of the anti-inflammatory effect of β-thujaplicin. Moreover, its capability of inhibiting inflammatory mediators e.g. TNF-alpha gene transcription, nitric oxide (NO) and prostaglandin E2, remains unknown. Besides those molecular mechanisms behind the anti-inflammatory effect of β-thujaplicin, solid proof of its effectiveness in vivo has not yet been studied. In our study, in vitro effects of β-thujaplicin were verified on RAW 264.7 macrophages which were stimulated by LPS. Indomethacin was used as a positive control. The inducible NO production after stimulation was measured by Griess reagent. PGE2, IL-6 and TNF-alpha were measured by ELISA methods. Protein expressions of iNOS, COX2, and NF-kB were evaluated by Western blotting. Septic ICR mice were administered 20 mg/kg of LPS and then the mortality rate was monitored. Within the concentration range which was devoid of cytotoxicty, β-thujaplicin exhibited a clear dose-dependent inhibition on LPS-induced NO production. Furthermore, β-thujaplicin inhibited LPS-induced PGE2, IL-6, and TNF-alpha production as well as iNOS, COX2, and NF-kB protein expression more substantially potent than indomethacin. In agreement with the in vitro study, β-thujaplicin was shown to be effective in vivo for inhibiting LPS-induced NO and TNF-alpha production and a significant decrease in mortality rate of mice suffering from septic shock was observed. This study demonstrates the potential of β-thujaplicin in treatment of inflammation and sepsis. These effects occur through an efficient blockage of TNF-alpha and iNOS production. β-thujaplicin efficacy is comparable to that of indomethacin thus it can be a substitution but bear less depletion of PGE2, making this compound very promising in clinical applications.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
Hinokitiol
CAS:499-44-5 |
C10H12O2 | |
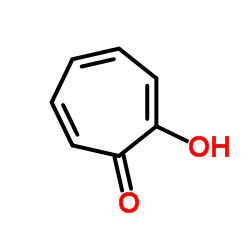 |
Tropolone
CAS:533-75-5 |
C7H6O2 |
|
Determination of hinokitiol in skin lotion by high-performan...
2013-01-01 [J. Cosmet. Sci. 64(5) , 381-9, (2013)] |
|
The furan route to tropolones: probing the antiproliferative...
2012-11-21 [Org. Biomol. Chem. 10(43) , 8597-604, (2012)] |
|
High-performance liquid chromatography with dual-wavelength ...
2009-01-01 [J. Cosmet. Sci. 60(5) , 519-25, (2009)] |
|
Volatile and non-volatile monoterpenes produced by elicitor-...
2009-05-01 [J. Plant Physiol. 166(7) , 720-8, (2009)] |
|
Preventive effects of β-thujaplicin against UVB-induced MMP-...
2012-01-01 [Am. J. Chin. Med. 40(2) , 387-98, (2012)] |