Tropolone
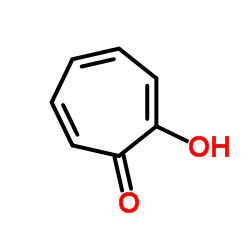
Tropolone structure
|
Common Name | Tropolone | ||
|---|---|---|---|---|
| CAS Number | 533-75-5 | Molecular Weight | 122.121 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 290.1±33.0 °C at 760 mmHg | |
| Molecular Formula | C7H6O2 | Melting Point | 50-52 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 122.0±18.0 °C | |
Use of TropoloneTropolone, a tropone derivative with a hydroxyl group in the 2-position, is a precursor of manyazulene derivatives such as methyl 2-methylazulene-1-carboxylate[1]. Tropolone is a potent inhibitor of mushroom tyrosinase with a IC50 of 0.4 μM, and the inhibition can be reversed by dialysis or by excess CU2+[2]. |
| Name | Tropolone |
|---|---|
| Synonym | More Synonyms |
| Description | Tropolone, a tropone derivative with a hydroxyl group in the 2-position, is a precursor of manyazulene derivatives such as methyl 2-methylazulene-1-carboxylate[1]. Tropolone is a potent inhibitor of mushroom tyrosinase with a IC50 of 0.4 μM, and the inhibition can be reversed by dialysis or by excess CU2+[2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.4 μM (mushroom tyrosinase)[2] |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 290.1±33.0 °C at 760 mmHg |
| Melting Point | 50-52 °C(lit.) |
| Molecular Formula | C7H6O2 |
| Molecular Weight | 122.121 |
| Flash Point | 122.0±18.0 °C |
| Exact Mass | 122.036781 |
| PSA | 37.30000 |
| LogP | 0.42 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.603 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | R22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | GU4075000 |
| HS Code | 29144090 |
| Precursor 7 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914400090 |
|---|---|
| Summary | 2914400090 other ketone-alcohols and ketone-aldehydes。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |
|
Identifying chelators for metalloprotein inhibitors using a fragment-based approach.
J. Med. Chem. 54 , 591-602, (2011) Fragment-based lead design (FBLD) has been used to identify new metal-binding groups for metalloenzyme inhibitors. When screened at 1 mM, a chelator fragment library (CFL-1.1) of 96 compounds produced... |
|
|
Determination of hinokitiol in skin lotion by high-performance liquid chromatography-ultraviolet detection after precolumn derivatization with 4-fluoro-7-nitro-2,1,3-benzoxadiazole.
J. Cosmet. Sci. 64(5) , 381-9, (2013) Hinokitiol, a potent, broad-spectrum antibacterial agent, is a component of various personal care products. In this study, the concentration of hinokitiol in skin lotion was analyzed by means of high-... |
|
|
The furan route to tropolones: probing the antiproliferative effects of β-thujaplicin analogs.
Org. Biomol. Chem. 10(43) , 8597-604, (2012) A direct route to analogs of the naturally occurring tropolone β-thujaplicin has been developed in just four steps from furan. Using this method, a series of derivatives were synthesized and evaluated... |
| 2-hydroxycyclohepta-2,4,6-trien-1-one |
| 2-Hydroxy-2,4,6-cycloheptatrienone |
| 2-hydroxy-2,4,6-cycloheptatriene-1-one |
| Purpurocatechol |
| 2,4,6-Cycloheptatrien-1-one, 2-hydroxy- |
| 2-hydroxytropone |
| 2-hydroxi-2,4,6-cycloheptatrien-1-one |
| EINECS 208-577-2 |
| TROPOLONE FOR SYNTHESIS 5 G |
| Tropolone |
| a-Tropolone |
| 2-hydroxycyclohepta-2,4,6-trienone |
| TROPOLONE FOR SYNTHESIS 1 G |
| MFCD00004158 |
| 2-Hydroxy-2,4,6-cycloheptatrien-1-one |
 CAS#:5307-99-3
CAS#:5307-99-3 CAS#:50904-09-1
CAS#:50904-09-1 CAS#:87563-17-5
CAS#:87563-17-5 CAS#:87563-16-4
CAS#:87563-16-4 CAS#:87563-19-7
CAS#:87563-19-7 CAS#:77367-72-7
CAS#:77367-72-7 CAS#:77367-70-5
CAS#:77367-70-5 CAS#:38768-08-0
CAS#:38768-08-0![Methyl 2-oxo-2H-cyclohepta[b]furan-3-carboxylate structure](https://image.chemsrc.com/caspic/021/50603-71-9.png) CAS#:50603-71-9
CAS#:50603-71-9 CAS#:2297-94-1
CAS#:2297-94-1 CAS#:21505-25-9
CAS#:21505-25-9 CAS#:20217-99-6
CAS#:20217-99-6 CAS#:22704-53-6
CAS#:22704-53-6 CAS#:2745-05-3
CAS#:2745-05-3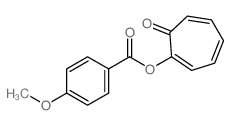 CAS#:2961-86-6
CAS#:2961-86-6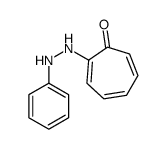 CAS#:2745-07-5
CAS#:2745-07-5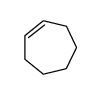 CAS#:628-92-2
CAS#:628-92-2
