| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sodium hydroxide
CAS:1310-73-2 |
|
 |
3-Ethyl-2,4-pentanedione
CAS:1540-34-7 |
|
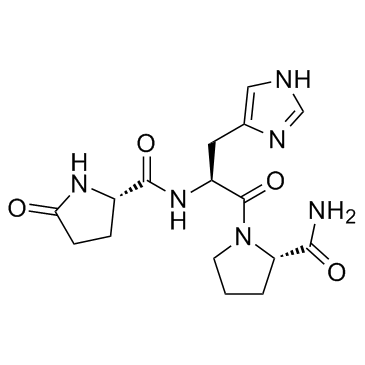 |
TRH
CAS:24305-27-9 |