| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sodium hydroxide
CAS:1310-73-2 |
|
 |
3-Ethyl-2,4-pentanedione
CAS:1540-34-7 |
|
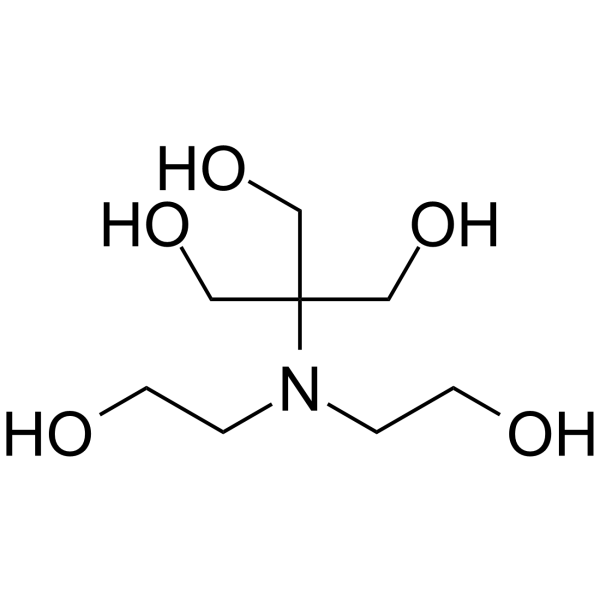 |
Bis-tris methane
CAS:6976-37-0 |
|
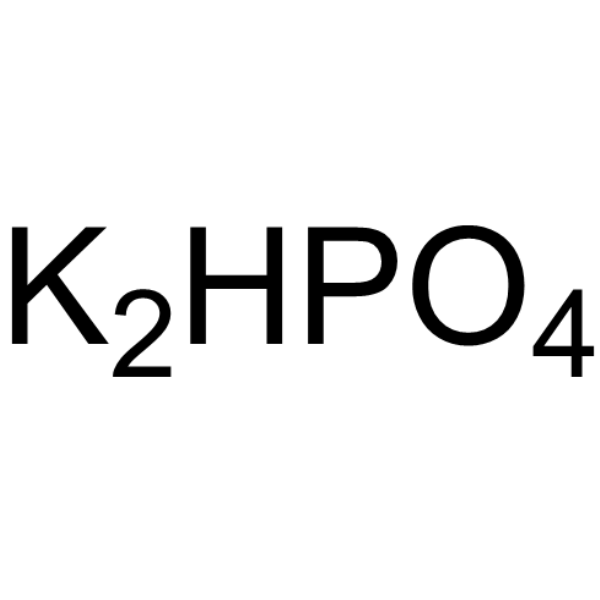 |
Di-potassium monohydrogen phosphate
CAS:7758-11-4 |
|
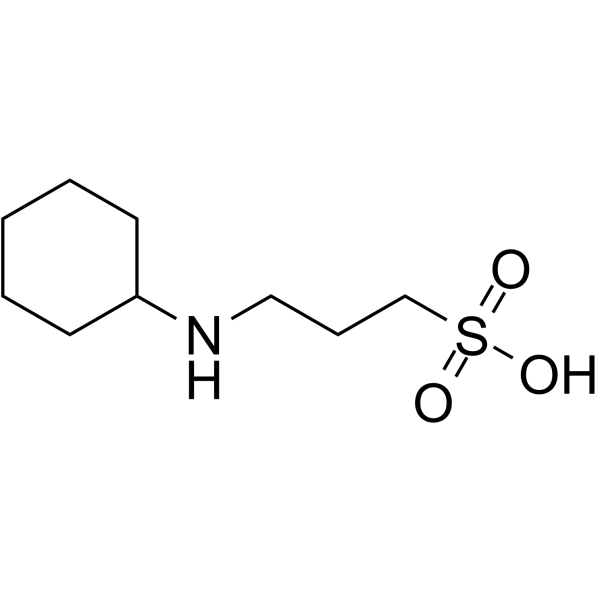 |
N-Cyclohexyl-3-aminopropanesulfonic acid
CAS:1135-40-6 |