| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sucrose
CAS:57-50-1 |
|
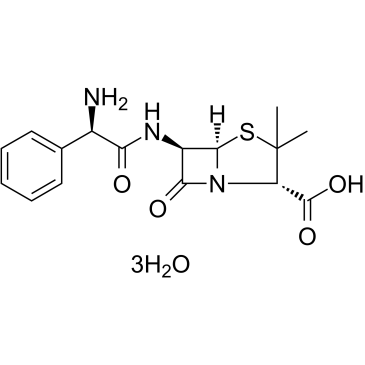 |
Ampicillin Trihydrate
CAS:7177-48-2 |
|
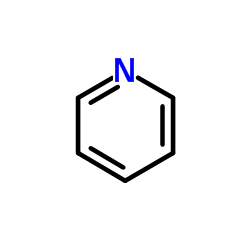 |
Pyridine
CAS:110-86-1 |
|
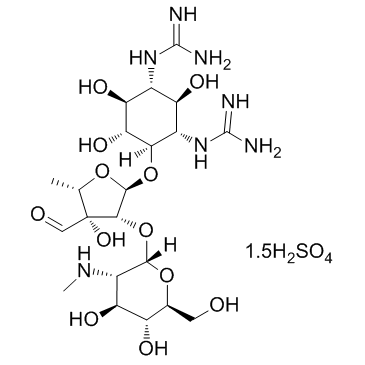 |
Steptomycin sulfate
CAS:3810-74-0 |
|
 |
Ethyl isocyanate
CAS:109-90-0 |