Steptomycin sulfate
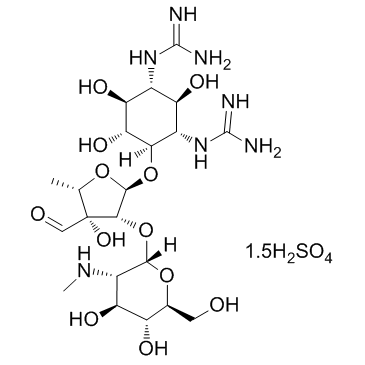
Steptomycin sulfate structure
|
Common Name | Steptomycin sulfate | ||
|---|---|---|---|---|
| CAS Number | 3810-74-0 | Molecular Weight | 728.69 | |
| Density | N/A | Boiling Point | 948.2ºC at 760 mmHg | |
| Molecular Formula | C21H42N7O18S1.5 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 527.3ºC | |
| Symbol |


GHS07, GHS08 |
Signal Word | Warning | |
Use of Steptomycin sulfateStreptomycin sulfate is an aminoglycoside antibiotic, that inhibits protein synthesis. |
| Name | streptomycin sesquisulfate |
|---|---|
| Synonym | More Synonyms |
| Description | Streptomycin sulfate is an aminoglycoside antibiotic, that inhibits protein synthesis. |
|---|---|
| Related Catalog | |
| In Vitro | Strain RB1 shows enhanced susceptibility to streptomycin as the concentration of CV in the growth medium increases. As the CV concentration in the growth medium increases, both cytochrome aa3 levels and streptomycin susceptibility increase. Cytochrome aa3 is necessary for accumulation of streptomycin by B. subtilis[1]. Streptomycin influences tRNA selection. Streptomycin resistance mutations generally map to protein S12 and most of these variants exhibit increased levels of discrimination in the tRNA selection process[2]. |
| References |
| Boiling Point | 948.2ºC at 760 mmHg |
|---|---|
| Molecular Formula | C21H42N7O18S1.5 |
| Molecular Weight | 728.69 |
| Flash Point | 527.3ºC |
| PSA | 911.80000 |
| Index of Refraction | -85 ° (C=1, H2O) |
| InChIKey | QTENRWWVYAAPBI-YZTFXSNBSA-N |
| SMILES | CNC1C(OC2C(OC3C(O)C(O)C(N=C(N)N)C(O)C3N=C(N)N)OC(C)C2(O)C=O)OC(CO)C(O)C1O.CNC1C(OC2C(OC3C(O)C(O)C(N=C(N)N)C(O)C3N=C(N)N)OC(C)C2(O)C=O)OC(CO)C(O)C1O.O=S(=O)(O)O.O=S(=O)(O)O.O=S(=O)(O)O |
| Storage condition | 2-8°C |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| Water Solubility | >=0.01 G/100 ML AT 18 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H361 |
| Precautionary Statements | P281 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| Safety Phrases | 26-36 |
| RIDADR | 2811.0 |
| WGK Germany | 3 |
| RTECS | WK4990000 |
| Hazard Class | 6.1 |
| HS Code | 2941200000 |
| HS Code | 2941200000 |
|---|
|
A precisely substituted benzopyran targets androgen refractory prostate cancer cells through selective modulation of estrogen receptors.
Toxicol. Appl. Pharmacol. 283(3) , 187-97, (2015) Dietary consumption of phytoestrogens like genistein has been linked with lower incidence of prostate cancer. The estradiol-like benzopyran core of genistein confers estrogen receptor-β (ER-β) selecti... |
|
|
Involvement of epigenetics and EMT-related miRNA in arsenic-induced neoplastic transformation and their potential clinical use.
Cancer Prev. Res. (Phila.) 8(3) , 208-21, (2015) Exposure to toxicants leads to cumulative molecular changes that overtime increase a subject's risk of developing urothelial carcinoma. To assess the impact of arsenic exposure at a time progressive m... |
|
|
Nitric oxide donors reduce the invasion ability of ovarian cancer cells in vitro.
Anticancer Drugs 25(10) , 1141-51, (2014) The most important factors involved in tumor metastasis and angiogenesis are metalloproteinases (MMPs), vascular endothelial growth factor, and multifunctional transforming growth factor β1. These fac... |
| N,N'-[(1R,2R,3S,4R,5R,6S)-4-[[5-deoxy-2-O-[2-deoxy-2-(methylamino)-α-L-glucopyranosyl]-3-C-formyl-α-L-lyxofuranosyl]oxy]-2,5,6-trihydroxy-1,3-cyclohexanediyl]bis[guanidine] sulfate (2:3) |
| O-2-deoxy-2-methylamino-α-L-glucopyranosyl-(1→2)-O-5-deoxy-3-C-formyl-α-L-lyxofuranosyl-(1→4)-N1,N3-diamidino-D-streptamine sulfate (2:3) (salt) |
| MFCD00037023 |
| Streptomycin sulfate salt |
| Streptomycin Sulfate |
| EINECS 223-286-0 |
| Streptomycin Sulphate |
| 1,1’-{1-D-(1,3,5/2,4,6)-4-[5-deoxy-2-O-(2-deoxy-2-methylamino-α-L-glucopyranosyl)-3-C-formyl-α-L-lyxofuranosyloxy]-2,5,6-trihydroxycyclohex-1,3-ylene}diguanidine sulfate (2:3) (salt) |
| Streptomycin (sulfate) |

