| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium chloride
CAS:7647-14-5 |
|
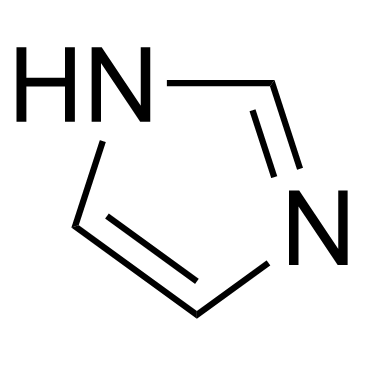 |
Imidazole
CAS:288-32-4 |
|
 |
HEPES
CAS:7365-45-9 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
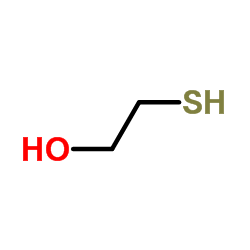 |
mercaptoethanol
CAS:60-24-2 |
|
 |
Glycerol
CAS:56-81-5 |
|
 |
Praseodymium
CAS:7440-10-0 |
|
 |
Ethylenediaminetetraacetic acid
CAS:60-00-4 |