| Structure | Name/CAS No. | Articles |
|---|---|---|
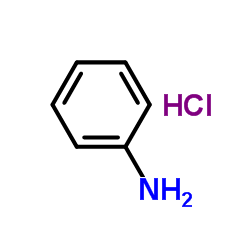 |
Anilinium chloride
CAS:142-04-1 |
|
 |
Aniline
CAS:62-53-3 |
|
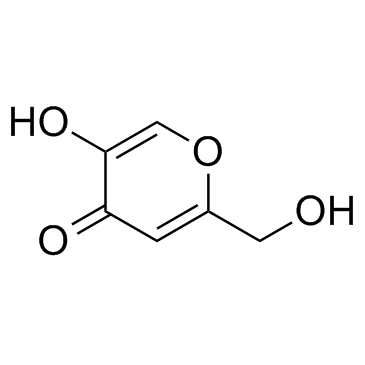 |
kojic acid
CAS:501-30-4 |
|
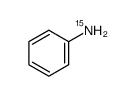 |
Aniline-15N
CAS:7022-92-6 |
|
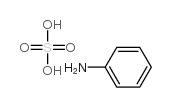 |
Aniline sulfate
CAS:542-16-5 |