| Structure | Name/CAS No. | Articles |
|---|---|---|
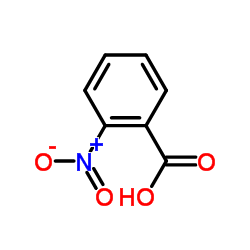 |
2-Nitrobenzoic acid
CAS:552-16-9 |
|
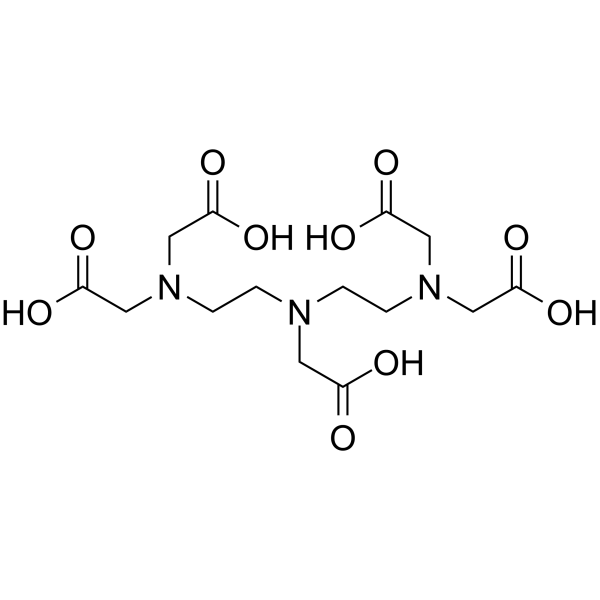 |
Diethylenetriaminepentaacetic acid
CAS:67-43-6 |
|
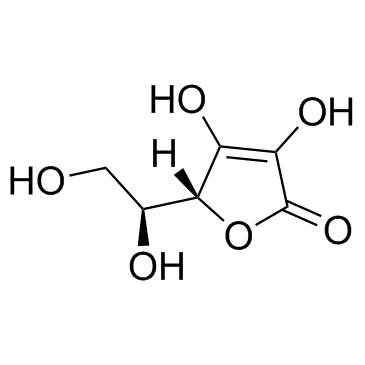 |
Ascorbic acid
CAS:50-81-7 |
|
 |
Fructose
CAS:57-48-7 |
|
 |
Dihydrorhodamine 123
CAS:109244-58-8 |
|
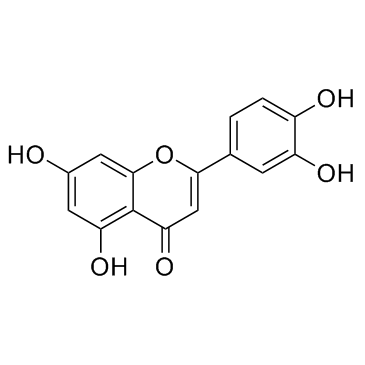 |
Luteolin
CAS:491-70-3 |
|
 |
PMSF
CAS:329-98-6 |
|
 |
Ethylenediaminetetraacetic acid
CAS:60-00-4 |
|
 |
Acetylthiocholine Iodide
CAS:1866-15-5 |
|
 |
Quercetin dihydrate
CAS:6151-25-3 |