| Structure | Name/CAS No. | Articles |
|---|---|---|
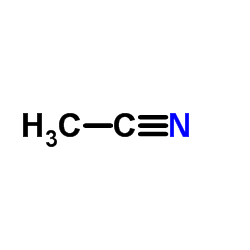 |
Acetonitrile
CAS:75-05-8 |
|
 |
Methanol
CAS:67-56-1 |
|
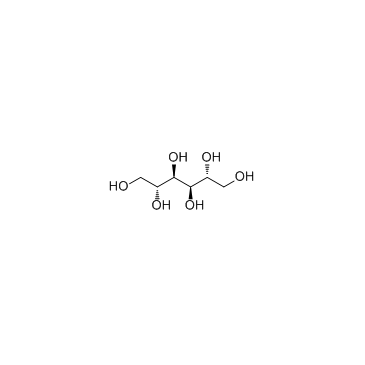 |
D-Mannitol
CAS:69-65-8 |
|
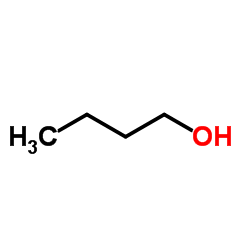 |
Butanol
CAS:71-36-3 |
|
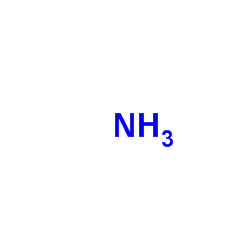 |
Ammonia
CAS:7664-41-7 |
|
 |
AMMONIA (14N)
CAS:1026405-88-8 |
|
 |
Gliclazide
CAS:21187-98-4 |
|
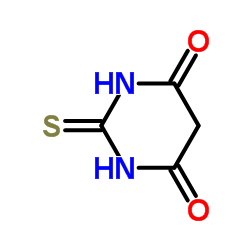 |
4,6-Dihydroxy-2-mercaptopyrimidine
CAS:504-17-6 |