| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
N,N-Dimethylformamide
CAS:68-12-2 |
|
 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
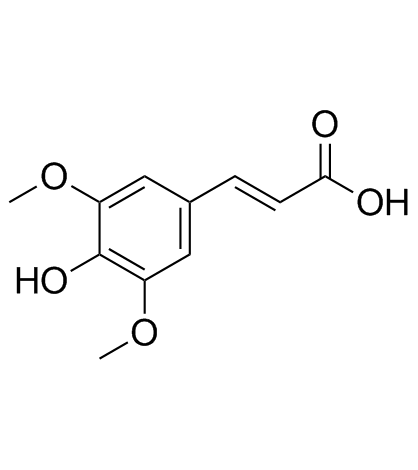 |
Sinapic acid
CAS:530-59-6 |
|
 |
trifluoroacetic acid
CAS:76-05-1 |
|
 |
4-Nitrophenol
CAS:100-02-7 |
|
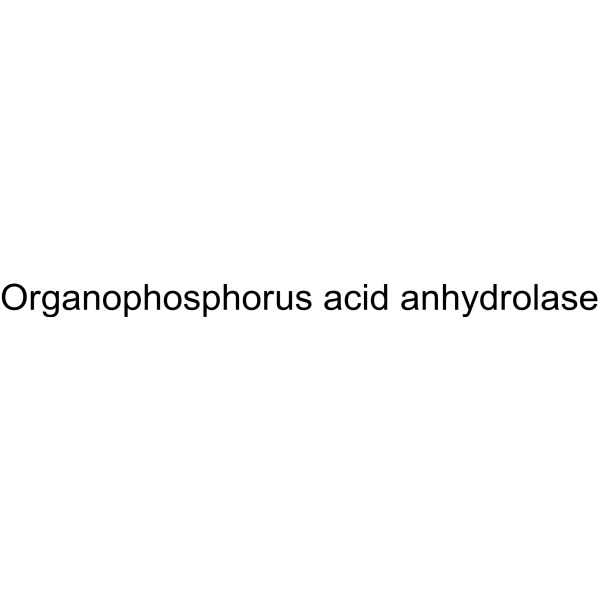 |
Diisopropylfluoro-phosphatase, lyo
CAS:9032-18-2 |
|
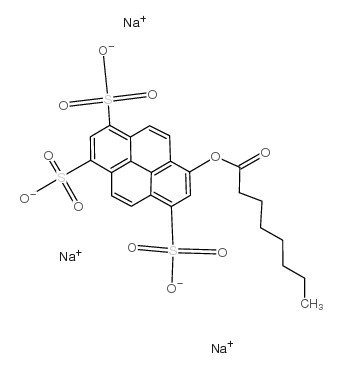 |
8-Octanoyloxypyrene-1,3,6-trisulfonic acid trisodium salt
CAS:115787-84-3 |