Relationship between substrate activity and pKa value of phenols on sulfotransferase from Eubacterium A-44.
L Konishi-Imamura, D H Kim, K Kobashi
Index: Biochem. Int. 28(4) , 725-34, (1992)
Full Text: HTML
Abstract
The relationship between the kinetics of the enzyme activity and the structural features of phenolic donor and of acceptor substrates was investigated with a sulfotransferase from Eubacterium A-44, a human intestinal bacterium. The enzyme catalyzed the transfer of the sulfate group from the sulfate esters of phenol having a lower pKa to phenols having a higher pKa. When the Km values for acceptor substrates were measured at their optimal pH, a linear plot for log10Km versus the pKa with a slope of 0.615 was obtained. In addition, it is considered that the effect of pH on the Km values for the various acceptors is due to ionization of free enzyme. The kinetic behavior of bacterial sulfotransferase differed from that of mammalian phenol sulfotransferase.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
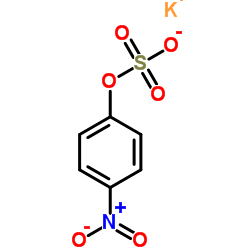 |
Potassium 4-nitrophenyl sulfate
CAS:6217-68-1 |
C6H4KNO6S |
|
Regulation of arylsulfate sulfotransferase from a human inte...
1995-01-01 [J. Enzym. Inhib. 8(4) , 233-41, (1995)] |
|
Sulfating-activity and stability of cDNA-expressed allozymes...
1999-08-01 [J. Biochem. 126(2) , 271-7, (1999)] |
|
Kinetic studies on a novel sulfotransferase from Eubacterium...
1991-01-01 [J. Biochem. 109(1) , 45-8, (1991)] |
|
para-Nitrophenyl sulfate activation of human sulfotransferas...
2009-10-23 [J. Biol. Chem. 284(43) , 29357-64, (2009)] |
|
Natural killer cell cytolytic granule-associated enzymes. I....
1991-08-01 [J. Immunol. 147(3) , 950-8, (1991)] |