Regulation of arylsulfate sulfotransferase from a human intestinal bacterium by nucleotides and magnesium ion.
L Konishi-Imamura, D H Kim, M Koizumi, K Kobashi
Index: J. Enzym. Inhib. 8(4) , 233-41, (1995)
Full Text: HTML
Abstract
Arylsulfate sulfotransferase (ASST) from a human intestinal bacterium stoichiometrically catalyzed the transfer of a sulfate group from phenylsulfate esters to phenolic compounds. Pentachlorophenol, one of the selective inhibitors of phenol sulfoconjugation in mammalian tissues, inhibited both phenol and tyramine sulfation by ASST. Nucleotide triphosphates such as ATP, GTP, UTP and CTP, and pyrophosphate inhibited the ASST activity, whereas Mg2+ and Mn2+ activated the enzyme and prevented its inhibition by ATP and pyrophosphate. Equimolar binding of [alpha-] and [gamma-32P]ATP to the enzyme showed that the enzyme protein was not phosphorylated, but bound ATP. These results suggest that nucleotide triphosphates and divalent cations are important modulators in the control of ASST activity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
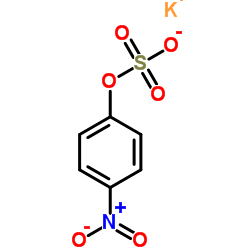 |
Potassium 4-nitrophenyl sulfate
CAS:6217-68-1 |
C6H4KNO6S |
|
Relationship between substrate activity and pKa value of phe...
1992-12-01 [Biochem. Int. 28(4) , 725-34, (1992)] |
|
Sulfating-activity and stability of cDNA-expressed allozymes...
1999-08-01 [J. Biochem. 126(2) , 271-7, (1999)] |
|
Kinetic studies on a novel sulfotransferase from Eubacterium...
1991-01-01 [J. Biochem. 109(1) , 45-8, (1991)] |
|
para-Nitrophenyl sulfate activation of human sulfotransferas...
2009-10-23 [J. Biol. Chem. 284(43) , 29357-64, (2009)] |
|
Natural killer cell cytolytic granule-associated enzymes. I....
1991-08-01 [J. Immunol. 147(3) , 950-8, (1991)] |