Trimethylsilyl trifluoromethanesulfonate-promoted reductive 2'-O-arylmethylation of ribonucleoside derivatives.
Naoki Uchiyama, Toshihiko Ogata, Natsuhisa Oka, Takeshi Wada
Index: Nucleosides Nucleotides Nucleic Acids 30(6) , 446-56, (2011)
Full Text: HTML
Abstract
Arylmethyl groups such as benzyl, p-methoxybenzyl, and 1-pyrenylmethyl groups were introduced to the 2'-O-position of nucleosides by reductive etherification. Combining corresponding aromatic aldehydes with 2'-O-trimethylsilylnucleoside derivatives in the presence of trimethylsilyl trifluoromethanesulfonate (TMSOTf) resulted in moderate to good yields of the 2'-O-arylmethyluridine derivatives, whereas the corresponding cytidine and adenosine derivatives were obtained in low yields. The reaction of ribonucleosides with aliphatic aldehydes did not proceed smoothly. Anomerization of the uridine derivatives by TMSOTf was observed in CH(2)Cl(2), toluene, and CH(3)CN, but was completely suppressed when the reactions were conducted in 1,4-dioxane.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
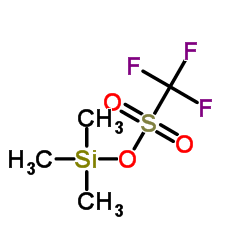 |
Trimethylsilyl trifluoromethanesulfonate
CAS:27607-77-8 |
C4H9F3O3SSi |
|
Trimethylsilyl trifluoromethanesulfonate (TMSOTf) assisted f...
2008-01-18 [J. Org. Chem. 73(2) , 752-5, (2008)] |
|
Nucleophilic substitution at the anomeric position of 1,2-O-...
2006-12-29 [Carbohydr. Res. 341(18) , 2883-90, (2006)] |
|
Intramolecular formal [4 + 2] cycloaddition of nitriles with...
2009-08-07 [J. Org. Chem. 74(15) , 5699-702, (2009)] |
|
Diastereoselective synthesis of substituted dihydropyrans vi...
2012-11-21 [Org. Biomol. Chem. 10(43) , 8730-8, (2012)] |
|
Facile synthesis of N-(1-alkenyl) derivatives of 2,4-pyrimid...
2000-07-01 [Nucleosides Nucleotides Nucleic Acids 19 , 1093-1100, (2000)] |