Trimethylsilyl trifluoromethanesulfonate (TMSOTf) assisted facile deprotection of N,O-acetonides.
Kevin W C Poon, Kimberly M Lovell, Kendra N Dresner, Apurba Datta
Index: J. Org. Chem. 73(2) , 752-5, (2008)
Full Text: HTML
Abstract
Employing TMSOTf as an easily available reagent, we have developed a mild and efficient method for the deprotection of both terminal and internal N,0-acetonide functionalities. Various regularly used protecting groups and common organic functional moieties were found to be unaffected by the described reaction conditions. In a few representative examples, the present method was also extended to deprotect acetonides obtained from 1,2-, and 1,3-terminal diols. The acetonide deprotection protocol described herein is expected to be a useful addition to the presently available methods for performing the above transformation.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
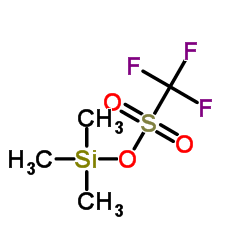 |
Trimethylsilyl trifluoromethanesulfonate
CAS:27607-77-8 |
C4H9F3O3SSi |
|
Trimethylsilyl trifluoromethanesulfonate-promoted reductive ...
2011-06-01 [Nucleosides Nucleotides Nucleic Acids 30(6) , 446-56, (2011)] |
|
Nucleophilic substitution at the anomeric position of 1,2-O-...
2006-12-29 [Carbohydr. Res. 341(18) , 2883-90, (2006)] |
|
Intramolecular formal [4 + 2] cycloaddition of nitriles with...
2009-08-07 [J. Org. Chem. 74(15) , 5699-702, (2009)] |
|
Diastereoselective synthesis of substituted dihydropyrans vi...
2012-11-21 [Org. Biomol. Chem. 10(43) , 8730-8, (2012)] |
|
Facile synthesis of N-(1-alkenyl) derivatives of 2,4-pyrimid...
2000-07-01 [Nucleosides Nucleotides Nucleic Acids 19 , 1093-1100, (2000)] |