Isolation and characterization of products from the nitrosation of the alkaloid gramine.
M U Ahmad, L M Libbey, J F Barbour, R A Scanlan
Index: Food Chem. Toxicol. 23(9) , 841-7, (1985)
Full Text: HTML
Abstract
The nitrosation of gramine, a tertiary amine alkaloid present in barley malt, was carried out by reaction with sodium nitrite in buffered acetic acid (pH 3.4) for 1 hr at room temperature. Two major non-volatile products of the nitrosation reaction were isolated by preparative HPLC and characterized as indole-3-carboxylic acid and N1-nitroso-3-nitromethylindole. This interpretation was supported by spectral data. The nature of these products indicated that gramine did not undergo nitrosation by the expected mechanism of nitrosative dealkylation. A mechanism is offered to explain the labile nature of the dimethylamino group found in gramine.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
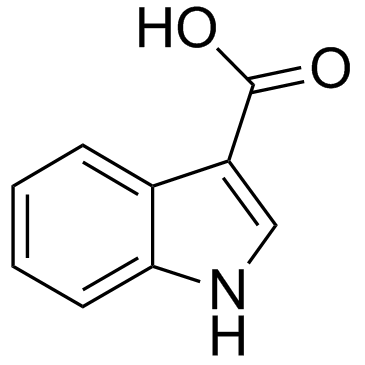 |
1H-Indole-3-carboxylic acid
CAS:771-50-6 |
C9H7NO2 |
|
QSAR study on permeability of hydrophobic compounds with art...
2007-06-01 [Bioorg. Med. Chem. 15 , 3756-67, (2007)] |
|
Total synthesis of kottamide E.
2013-03-21 [Chem. Commun. (Camb.) 49(23) , 2296-8, (2013)] |
|
Glycine conjugates in a lepidopteran insect herbivore--the m...
2006-12-01 [ChemBioChem. 7(12) , 1982-9, (2006)] |
|
Purification and biological evaluation of the metabolites pr...
2010-06-01 [Res. Microbiol. 161(5) , 335-45, (2010)] |
|
Novel reversible indole-3-carboxylate decarboxylase catalyzi...
2002-11-01 [Biosci. Biotechnol. Biochem. 66(11) , 2388-94, (2002)] |