Novel reversible indole-3-carboxylate decarboxylase catalyzing nonoxidative decarboxylation.
Toyokazu Yoshida, Kohei Fujita, Toru Nagasawa
Index: Biosci. Biotechnol. Biochem. 66(11) , 2388-94, (2002)
Full Text: HTML
Abstract
After enrichment culture with indole-3-carboxylate in static culture, a novel reversible decarboxylase, indole-3-carboxylate decarboxylase, was found in Arthrobacter nicotianae FI1612 and several molds. The enzyme reaction was examined in resting-cell reactions with A. nicotianae FI1612. The enzyme activity was induced specifically by indole-3-carboxylate, but not by indole. The indole-3-carboxylate decarboxylase of A. nicotianae FI1612 catalyzed the nonoxidative decarboxylation of indole-3-carboxylate into indole, and efficiently carboxylated indole and 2-methylindole by the reverse reaction. In the presence of 1 mM dithiothreitol, 50 mM Na2 S2O3, and 20% (v/v) glycerol, indole-3-carboxylate decarboxylase was partially purified from A. nicotianae FI1612. The purified enzyme had a molecular mass of approximately 258 kDa. The enzyme did not need any cofactor for the decarboxylating and carboxylating reactions.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
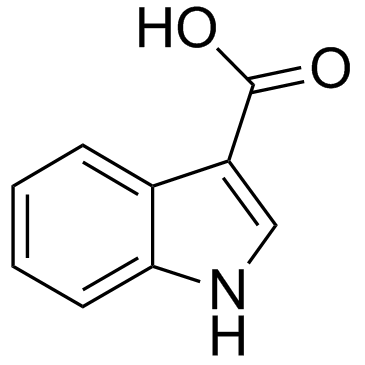 |
1H-Indole-3-carboxylic acid
CAS:771-50-6 |
C9H7NO2 |
|
QSAR study on permeability of hydrophobic compounds with art...
2007-06-01 [Bioorg. Med. Chem. 15 , 3756-67, (2007)] |
|
Isolation and characterization of products from the nitrosat...
1985-09-01 [Food Chem. Toxicol. 23(9) , 841-7, (1985)] |
|
Total synthesis of kottamide E.
2013-03-21 [Chem. Commun. (Camb.) 49(23) , 2296-8, (2013)] |
|
Glycine conjugates in a lepidopteran insect herbivore--the m...
2006-12-01 [ChemBioChem. 7(12) , 1982-9, (2006)] |
|
Purification and biological evaluation of the metabolites pr...
2010-06-01 [Res. Microbiol. 161(5) , 335-45, (2010)] |