| Structure | Name/CAS No. | Articles |
|---|---|---|
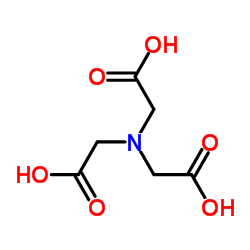 |
nitrilotriacetic acid
CAS:139-13-9 |
|
 |
Hemopexin
CAS:9013-71-2 |
|
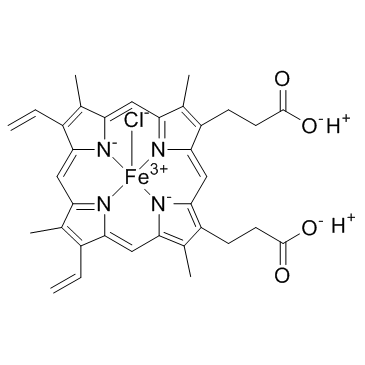 |
Hemin
CAS:16009-13-5 |