| Structure | Name/CAS No. | Articles |
|---|---|---|
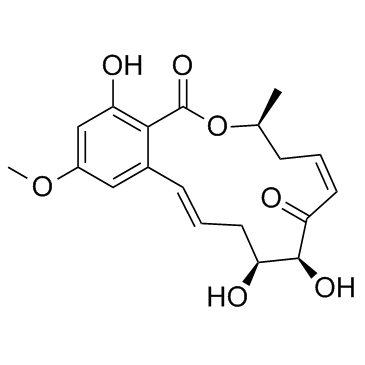 |
5Z-7-Oxozeaenol
CAS:253863-19-3 |
|
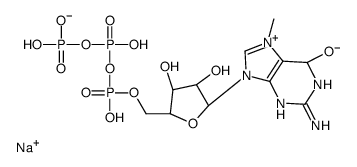 |
7-METHYLGUANOSINE 5'-TRIPHOSPHATE SODIUM SALT
CAS:104809-18-9 |