| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Forskolin
CAS:66575-29-9 |
|
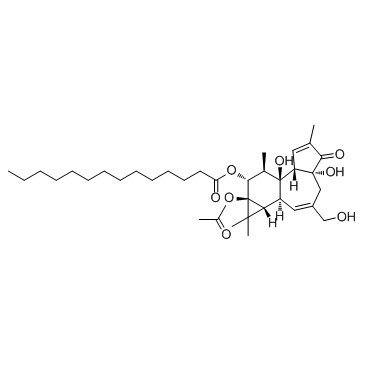 |
12-O-tetradecanoylphorbol-13-acetate
CAS:16561-29-8 |
|
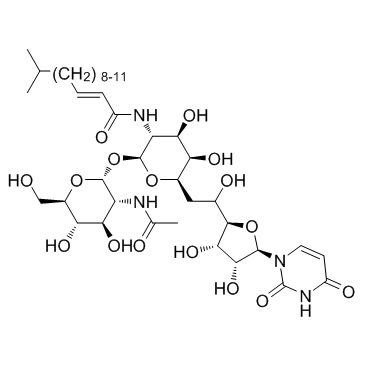 |
Tunicamycin
CAS:11089-65-9 |
|
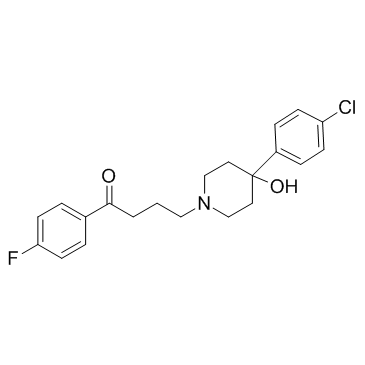 |
haloperidol
CAS:52-86-8 |