| Structure | Name/CAS No. | Articles |
|---|---|---|
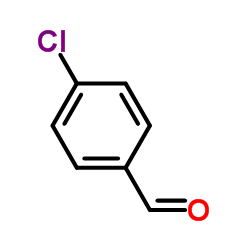 |
4-Chlorobenzaldehyde
CAS:104-88-1 |
|
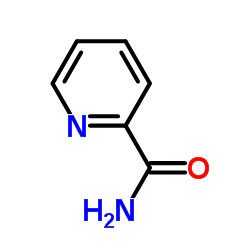 |
PYRIDINE-2-CARBOXAMIDE
CAS:1452-77-3 |
|
 |
4-Fluorobenzaldehyde
CAS:459-57-4 |