| Structure | Name/CAS No. | Articles |
|---|---|---|
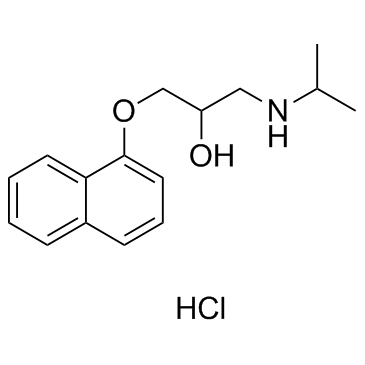 |
Propranolol hydrochloride
CAS:318-98-9 |
|
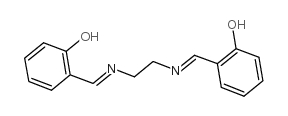 |
n,n'-bis(salicylidene)ethylenediamine
CAS:94-93-9 |