| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
L-(+)-Lysine monohydrochloride
CAS:657-27-2 |
|
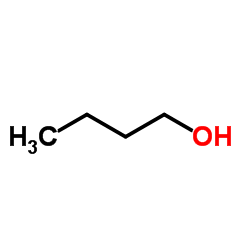 |
Butanol
CAS:71-36-3 |
|
 |
3-Isocyanatopropyltriethoxysilane
CAS:24801-88-5 |
|
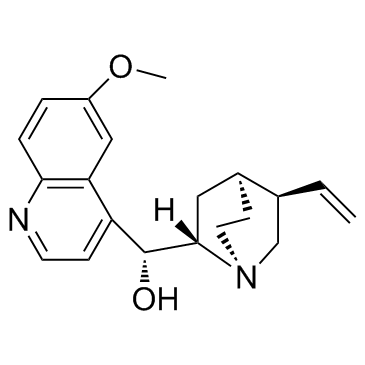 |
Quinine
CAS:130-95-0 |
|
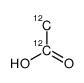 |
acetic acid
CAS:1173022-32-6 |
|
 |
acetic acid
CAS:64-19-7 |
|
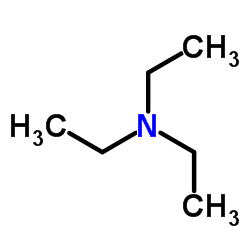 |
Triethylamine
CAS:121-44-8 |
|
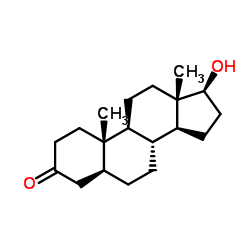 |
Stanolone
CAS:521-18-6 |