| Structure | Name/CAS No. | Articles |
|---|---|---|
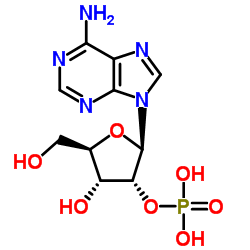 |
Adenosine-2'-monophosphate
CAS:130-49-4 |
|
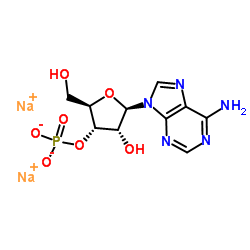 |
Adenosine-3'-monophosphate Sodium Salt
CAS:4958-39-8 |
|
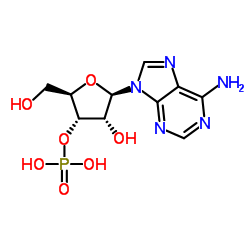 |
3'-Adenylic acid
CAS:84-21-9 |
|
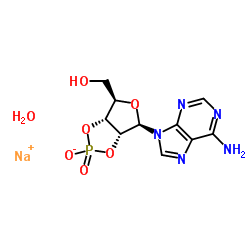 |
Adenosine, cyclic2',3'-(hydrogen phosphate), monosodium salt (9CI)
CAS:37063-35-7 |