| Structure | Name/CAS No. | Articles |
|---|---|---|
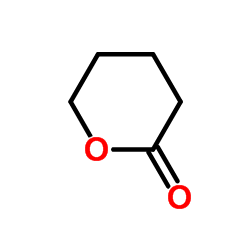 |
delta-Valerolactone
CAS:542-28-9 |
|
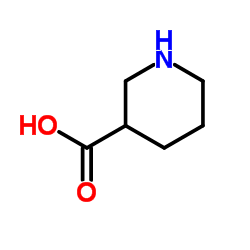 |
Nipecotic acid
CAS:498-95-3 |
|
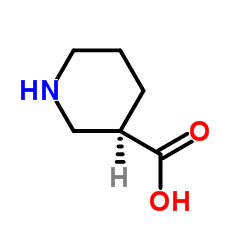 |
(R)-(-)-Nipecotic acid
CAS:25137-00-2 |
|
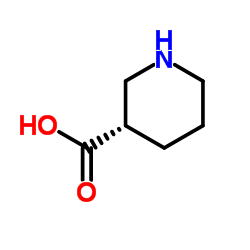 |
(S)-(+)-Nipecotic acid
CAS:59045-82-8 |