Nipecotic acid
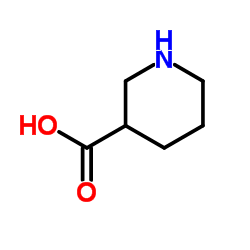
Nipecotic acid structure
|
Common Name | Nipecotic acid | ||
|---|---|---|---|---|
| CAS Number | 498-95-3 | Molecular Weight | 129.157 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 265.8±33.0 °C at 760 mmHg | |
| Molecular Formula | C6H11NO2 | Melting Point | 261ºC (dec.) | |
| MSDS | USA | Flash Point | 114.5±25.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain cancer. However, the complete repertoire of signaling pathways ... |
|
|
Discovery of {1-[4-(2-{hexahydropyrrolo[3,4-c]pyrrol-2(1H)-yl}-1H-benzimidazol-1-yl)piperidin-1-yl]cyclooctyl}methanol, systemically potent novel non-peptide agonist of nociceptin/orphanin FQ receptor as analgesic for the treatment of neuropathic pain: Design, synthesis, and structure–activity relationships
Bioorg. Med. Chem. 18 , 7675-99, (2010) Neuropathic pain is a serious chronic disorder caused by lesion or dysfunction in the nervous systems. Endogenous nociceptin/orphanin FQ (N/OFQ) peptide and N/OFQ peptide (NOP) receptor [or opioid-receptor-like-1 (ORL1) receptor] are located in the central an... |
|
|
Determination of plasma pipecolic acid by an easy and rapid liquid chromatography-tandem mass spectrometry method.
Clin. Chim. Acta 440 , 108-12, (2015) Pipecolic acid (PA) is an important biochemical marker for the diagnosis of peroxisomal disorders. PA is also a factor responsible for hepatic encephalopathy and a possible biomarker for pyridoxine-dependent seizures. We developed an easy and rapid PA quantif... |
|
|
Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila.
Nat. Chem. Biol. 4 , 256-63, (2008) Fragile X syndrome is caused by the functional loss of the fragile X mental retardation 1 (FMR1) gene. Deletion of the FMR1 ortholog in Drosophila melanogaster (Fmr1) recapitulates many phenotypes associated with fragile X syndrome. We have discovered that Fm... |
|
|
Protein prosthesis: β-peptides as reverse-turn surrogates.
Protein Sci. 22(3) , 274-9, (2013) The introduction of non-natural modules could provide unprecedented control over folding/unfolding behavior, conformational stability, and biological function of proteins. Success requires the interrogation of candidate modules in natural contexts. Here, expr... |
|
|
N-methyl phenylalanine-rich peptides as highly versatile blood-brain barrier shuttles.
J. Med. Chem. 53 , 2354-63, (2010) Here we studied the capacity of N-MePhe-(N-MePhe)(3)-CONH(2), Cha-(N-MePhe)(3)-CONH(2), and 2Nal-(N-MePhe)(3)-CONH(2) to carry various drugs (cargos) in in vitro blood-brain barrier (BBB) models in order to determine the versatility of these peptides as BBB-s... |
|
|
GABA uptake by purified avian Müller glia cells in culture.
Neurotox. Res. 12(2) , 145-53, (2007) GABA is the main inhibitory aminoacid transmitter present in neurons and glial cells. Its uptake is carried out by specific high-affinity Na(+)/Cl (-) dependent transporters (GATs). It has been reported in the past that, in the avian retina, [(3)H]GABA appear... |
|
|
Stereoselective synthesis of nipecotic acid derivatives via palladium-catalyzed decarboxylative cyclization of gamma-methylidene-delta-valerolactones with imines.
Org. Lett. 11(2) , 457-9, (2009) A new synthetic method of multisubstituted nipecotic acid (piperidine-3-carboxylic acid) derivatives has been developed by way of palladium-catalyzed decarboxylative cyclization of gamma-methylidene-delta-valerolactones with imines. By employing the diethoxyp... |
|
|
2-n-Butyl-9-methyl-8-[1,2,3]triazol-2-yl-9H-purin-6-ylamine and analogues as A2A adenosine receptor antagonists. Design, synthesis, and pharmacological characterization.
J. Med. Chem. 48 , 6887-96, (2005) Two types of adenosine receptor ligands were designed, i.e., 9H-purine and 1H-imidazo[4,5-c]pyridines, to obtain selective A(2A) antagonists, and we report here their synthesis and binding affinities for the four adenosine receptor subtypes A(1), A(2A), A(2B)... |
|
|
Azetidine derivatives as novel γ-aminobutyric acid uptake inhibitors: Synthesis, biological evaluation, and structure–activity relationship
Eur. J. Med. Chem. 45 , 2453-66, (2010) In this study azetidine derivatives representing conformationally constrained GABA or β-alanine analogs were evaluated for their potency as GABA-uptake inhibitors. The study comprised derivatives substituted in 2- as well as in 3-position with either an aceti... |