(R)-(-)-Nipecotic acid
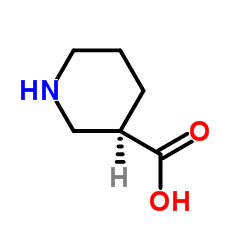
(R)-(-)-Nipecotic acid structure
|
Common Name | (R)-(-)-Nipecotic acid | ||
|---|---|---|---|---|
| CAS Number | 25137-00-2 | Molecular Weight | 129.157 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 265.8±33.0 °C at 760 mmHg | |
| Molecular Formula | C6H11NO2 | Melting Point | 251-255 °C | |
| MSDS | Chinese | Flash Point | 114.5±25.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Protein prosthesis: β-peptides as reverse-turn surrogates.
Protein Sci. 22(3) , 274-9, (2013) The introduction of non-natural modules could provide unprecedented control over folding/unfolding behavior, conformational stability, and biological function of proteins. Success requires the interrogation of candidate modules in natural contexts. Here, expr... |
|
|
N-methyl phenylalanine-rich peptides as highly versatile blood-brain barrier shuttles.
J. Med. Chem. 53 , 2354-63, (2010) Here we studied the capacity of N-MePhe-(N-MePhe)(3)-CONH(2), Cha-(N-MePhe)(3)-CONH(2), and 2Nal-(N-MePhe)(3)-CONH(2) to carry various drugs (cargos) in in vitro blood-brain barrier (BBB) models in order to determine the versatility of these peptides as BBB-s... |
|
|
GABA uptake by purified avian Müller glia cells in culture.
Neurotox. Res. 12(2) , 145-53, (2007) GABA is the main inhibitory aminoacid transmitter present in neurons and glial cells. Its uptake is carried out by specific high-affinity Na(+)/Cl (-) dependent transporters (GATs). It has been reported in the past that, in the avian retina, [(3)H]GABA appear... |
|
|
Stereoselective synthesis of nipecotic acid derivatives via palladium-catalyzed decarboxylative cyclization of gamma-methylidene-delta-valerolactones with imines.
Org. Lett. 11(2) , 457-9, (2009) A new synthetic method of multisubstituted nipecotic acid (piperidine-3-carboxylic acid) derivatives has been developed by way of palladium-catalyzed decarboxylative cyclization of gamma-methylidene-delta-valerolactones with imines. By employing the diethoxyp... |
|
|
Azetidine derivatives as novel γ-aminobutyric acid uptake inhibitors: Synthesis, biological evaluation, and structure–activity relationship
Eur. J. Med. Chem. 45 , 2453-66, (2010) In this study azetidine derivatives representing conformationally constrained GABA or β-alanine analogs were evaluated for their potency as GABA-uptake inhibitors. The study comprised derivatives substituted in 2- as well as in 3-position with either an aceti... |
|
|
An efficient lipase-catalyzed enantioselective hydrolysis of (R,S)-azolides derived from N-protected proline, pipecolic acid, and nipecotic acid.
Appl. Microbiol. Biotechnol. 97(4) , 1581-7, (2013) In the Candida antarctica lipase B-catalyzed hydrolysis of (R,S)-azolides derived from (R,S)-N-protected proline in water-saturated methyl tert-butyl ether (MTBE), high enzyme activity with excellent enantioselectivity (V (S) V (R) (-1) > 100) for (R,S)-N-Cb... |
|
|
Extrasynaptic GABA(A) receptors couple presynaptic activity to postsynaptic inhibition in the somatosensory thalamus.
J. Neurosci. 33(37) , 14850-68, (2013) Thalamocortical circuits govern cognitive, sensorimotor, and sleep-related network processes, and generate pathological activities during absence epilepsy. Inhibitory control of thalamocortical (TC) relay neurons is partially mediated by GABA released from ne... |
|
|
Parawixin2, a novel non-selective GABA uptake inhibitor from Parawixia bistriata spider venom, inhibits pentylenetetrazole-induced chemical kindling in rats.
Neurosci. Lett. 543 , 12-6, (2013) The aims of the present work were to investigate the effects of the repeated administration of Parawixin2 (2-amino-5-ureidopentanamide; formerly FrPbAII), a novel GABA and glycine uptake inhibitor, in rats submitted to PTZ-induced kindling. Wistar rats were r... |
|
|
A steered molecular dynamics study of binding and translocation processes in the GABA transporter.
PLoS ONE 7(6) , e39360, (2012) The entire substrate translocation pathway in the human GABA transporter (GAT-1) was explored for the endogenous substrate GABA and the anti-convulsive drug tiagabine. Following a steered molecular dynamics (SMD) approach, in which a harmonic restraining pote... |
|
|
Selective amino acid substitutions convert the creatine transporter to a gamma-aminobutyric acid transporter.
J. Biol. Chem. 282(21) , 15528-33, (2007) The creatine transporter (CRT) is a member of a large family of sodium-dependent neurotransmitter and amino acid transporters. The CRT is closely related to the gamma-aminobutyric acid (GABA) transporter, GAT-1, yet GABA is not an effective substrate for the ... |