| Structure | Name/CAS No. | Articles |
|---|---|---|
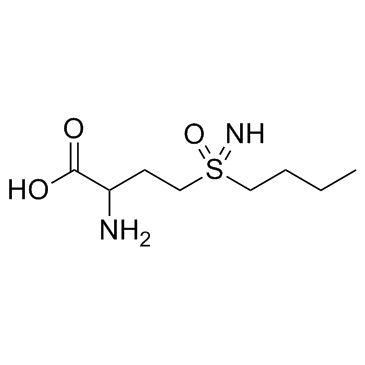 |
D,L-Buthionine-(S,R)-sulfoximine
CAS:5072-26-4 |
|
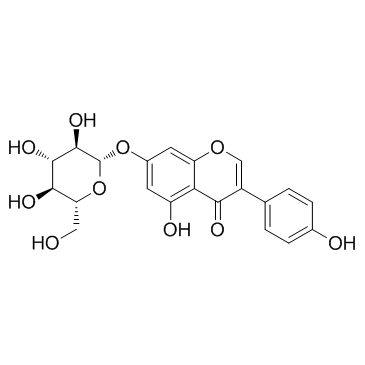 |
Genistin
CAS:529-59-9 |
|
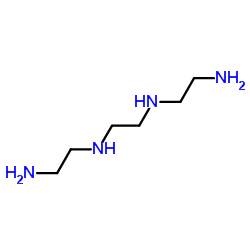 |
Triethylenetetramine
CAS:112-24-3 |